Figure 2.
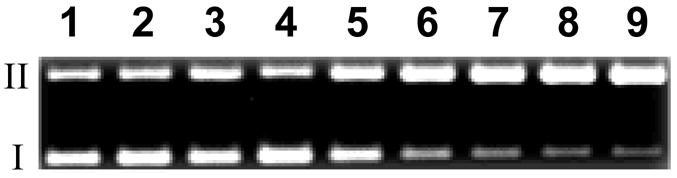
DNA cleavage by various concentrations of reductively-activated TPZ (1) under anaerobic conditions. Supercoiled plasmid DNA (33 μg/mL, pGL-2 Basic) was incubated with TPZ (25–150 μM), NADPH (500 μM), cytochrome P450 reductase (0.03 mU/μL), catalase (100 μg/mL), superoxide dismutase (10 μg/mL), sodium phosphate buffer (50 mM, pH 7.0), and desferal (1 mM) under anaerobic conditions at 25 °C for 4 h, followed by agarose gel electrophoretic analysis. Lane 1, DNA alone (S = 0.24 ± 0.01); lane 2, NADPH (500 μM) + reductase (0.03 mU/μL) (S = 0.26 ± 0.03); lane 3, TPZ (150 μM) (S = 0.24 ± 0.02); lanes 4–9, NADPH (500 μM) + reductase (0.03 mU/μL) + TPZ (25 μM, lane 4) (S = 0.38 ± 0.01); (50 μM, lane 5) (S = 0.51 ± 0.03); (75 μM, lane 6) (S = 0.61 ± 0.03); (100 μM, lane 7) (S = 0.72 ± 0.02); (125 μM, lane 8) (S = 0.82 ± 0.05); (150 μM, lane 9) (S = 1.04 ± 0.01). The values, S, represent the mean number of strand breaks per plasmid molecule and were calculated using the equation S = −ln fI, where fI is the fraction of plasmid present in the supercoiled form I.
