Abstract
Hairless rats were topically treated with a combination of 10% curcumin and 3% ginger extract (or with each agent alone) for a 21-day period. Following this, the rats were treated topically with Temovate (corticosteroid) for an additional 15 days. At the end of the treatment period, superficial abrasion wounds were induced in the treated skin. Abrasion wounds healed more slowly in the skin of Temovate-treated rats than in skin of control animals. Healing was more rapid in skin of rats that had been pre-treated with either curcumin or ginger extract alone or with the combination of curcumin-ginger extract (along with Temovate) than in the skin of rats treated with Temovate and vehicle alone. Skin samples were obtained at the time of wound closure. Collagen production was increased and matrix metalloproteinase-9 production was decreased in the recently-healed skin from rats treated with the botanical preparation relative to rats treated with Temovate plus vehicle. In none of the rats was there any indication of skin irritation during the treatment phase or during wounding and repair. Taken together, these data suggest that a combination of curcumin and ginger extract might provide a novel approach to improving structure and function in skin and, concomitantly, reducing formation of non-healing wounds in “at-risk” skin.
Keywords: collagen, skin damage, wound-repair
INTRODUCTION
The skin deteriorates as a consequence of the natural aging process (1–3). Extended use of corticosteroids also causes skin damage as these agents dramatically inhibit collagen synthesis (4,5). Finally, metabolic diseases such as diabetes lead to extensive skin damage (6–8). In all of these conditions, collagen synthesis is decreased, and collagen-degrading matrix metalloproteinases (MMPs) are increased. Skin that has severe connective tissue deficits is prone to bruising. When damaged skin suffers minor abrasion-type injuries, the wounds heal more slowly. Often, such wounds do not heal at all, but go on to form chronic ulcers with devastating consequences (1–11).
All-trans retinoic acid (RA) is the only agent currently approved by the FDA for the purpose of skin-repair. Past studies have documented the capacity of RA to increase production of type I procollagen and decrease elaboration of several MMPs (12–14). Not surprisingly, a number of past studies have documented beneficial effects of RA and other biologically-active retinoids in wound healing models (15–18). Our own studies have shown that retinoid-pretreatment of “at-risk” skin improves the healing of subsequently-induced superficial abrasion wounds (19,20). While prophylactic use of RA as a wound-preventative is an attractive strategy for individuals with severely damaged skin, topical retinoid use elicits skin irritation in most individuals (21–23). Skin irritation is the major reason for noncompliance among retinoid users. Further, if irritation is too great, the inflammatory changes can counteract the beneficial effects on collagen metabolism or even increase the susceptibility of the skin to wounding.
Efforts to identify other agents with skin-repair potential are underway in several laboratories. In the present study we demonstrate that pretreatment of hairless rats with a combination of two natural agents – i.e., curcumin (major curcuminoid in turmeric) and a 6-gingerol-enriched extract of ginger root – improves healing of superficial abrasion wounds that are subsequently induced in corticosteroid-treated (at-risk) skin. This is accomplished in the absence of significant skin irritation. Both curcumin and 6-gingerol (either highly pure or in a ginger root extract) have a variety of effects on the skin that may contribute to improved wound healing. Gingerol, in particular, is known to have anti-oxidant and anti-inflammatory properties (24–28), and has been reported to promote new blood vessel formation (25). Curcumin has also been shown to possess anti-oxidant properties (29–31). Perhaps more importantly, curcumin promotes collagen production and reduces matrix metalloproteinase levels (32–34). While there is little information on the value of 6-gingerol as a wound-preventative (35), several studies have suggested the beneficial effects of curcumin as a wound-healing agent (29–34,36–38). Given the overlapping, but complementary, activities in these two preparations, we decided to assess them in combination for effects on skin structure / function.
MATERIALS AND METHODS
Hairless rats
Male CD hairless rats (250–300 g) were purchased from Charles River Laboratories (Portage, MI). Rats were housed in temperature and light-controlled rooms and given water and feed ad libitum. All studies involving animals were approved by the University of Michigan Committee on Use and Care of Animals.
Curcumin
The Indian spice turmeric (Curcumin longa) contains three major curcuminoids, namely, curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Curcumin is the most abundant of the three. The preparation of curcumin used here was obtained from LKT Laboratories, Inc. (St. Paul, MN). The concentration of curcumin in the preparation was 93.6% with other curcuminoids accounting for 5.7% of the total as determined by HPLC.
6-Gingerol-enriched ginger extract
The ginger extract used here was a viscous, amber substance obtained by CO2 extraction from pulverized ginger roots (Zingiber officinale) (Dalton Chemical Laboratories; Toronto, Ontario). 6-Gingerol constitutes approximately 32% of the extract on a per weight basis as determined by HPLC.
Topical treatment and abrasion wound model
The botanicals were dissolved in dimethyl sulfoxide (DMSO) at 10.0% for curcumin and 3% for the ginger extract. The concentrations chosen for the two botanicals were based on results of preliminary in vitro studies (not shown).
Hairless rats were treated daily for 21 days topically over the back and flank with 500 µl of solutions containing 10% curcumin, 3% ginger extract or the combination of the two. DMSO alone served as control. During the treatment phase, animals were examined daily for changes in the gross appearance of the skin. After the initial 21-day pretreatment period, rats were treated with Temovate cream along with the botanicals for an additional 15 day period. Temovate (0.05% solution of clobetasol propionate in cream base) was obtained from Glaxo Smith Kline (Philadelphia, PA). Temovate was applied in the morning and the botanicals were applied in the evening. At the end of the treatment period, abrasion wounding was performed as described previously (20). Briefly, under general anesthesia (ketamine/xylazine), paravertebral skin from the back and flanks was cleaned with 70% ethanol. A pre-measured circular area, approximately 6 cm in diameter, was scrubbed with a stiff-nylon bristle brush lightly wetted with acetone and concomitantly abraded with a piece of course sanding sponge. Abrasion was sufficient to remove the thin epidermis and the upper part of the subepithelial stroma. Oozing of fluid (with a small amount of blood) into the abraded area indicated that the appropriate degree of abrasion had been achieved. The degree of injury was designed to approximate abrasions that commonly occur after a minor scrape. Wounding was performed under sterile conditions in a laminar flow hood. Wound size was determined daily by measuring the X and Y-axis of the scabbed wound and calculating the area of the remaining scab.
At the time of wound closure, animals were sacrificed and duplicate 6 mm skin punches were collected from the wounded and healed skin area. One biopsy was fixed in 10% buffered formalin and used for histological analysis. The other biopsy was put in organ culture for 2 days. Briefly, the skin was cut into pieces of approximately 2 mm on a side, and the pieces were incubated for 3 days in 0.5 ml of a culture medium consisting of growth factor-free, serum-free, Keratinocyte Basal Medium (KBM) (Lonza, Walkersville, MD). Before use, the culture medium was supplemented with Ca2+ to a final concentration of 1.55 mM. At the end of the 2-day incubation period, the organ culture fluid was collected and assayed for soluble type I collagen by western blotting and MMP-2 and -9 gelatin zymography (see below). The organ culture protocol was as described in a previous report (39).
Soluble type I collagen
Organ culture fluids were assayed for type I collagen by Western blot analysis (20). Briefly, organ culture fluids representing an equal quantity of protein were resolved using 8% SDS-PAGE under non-reducing conditions and transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) for 1 hour at room temperature. Following this, they were incubated overnight with a rabbit antibody to rodent type I collagen (1:10,000 dilution) (Abcam Inc., Cambridge, MA) in the same buffer at 4°C. The membranes were then washed with TTBS and bound antibody was detected using the Phototope-HRP western detection kit (Cell Signaling Technologies, Inc., Danvers, MA). Images were scanned, digitized, and quantified using NIH image analysis software.
MMP-2 and MMP-9
Gelatin-embedded enzymography (zymography) was used to assess levels of latent and active MMP-2 and MMP-9 in organ culture fluids as described previously (20). SDS-PAGE gels were prepared with the incorporation of gelatin (1 mg/ml) at the time of casting. After electrophoresis under non-reducing conditions to separate proteins and overnight incubation to allow for substrate digestion, zones of hydrolysis were identified as “holes” in the stained gels. Values for latent and active MMP-2 and -9 bands were obtained following digitization and quantification.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni posttest for selected pairs (GraphPad Prism Version 4.00 for Windows, GraphPad Software, San Diego, CA). Data were considered significant at p < 0.05.
RESULTS
Topical treatment with curcumin and ginger extract increases type I collagen and decreases MMP-9 in the skin of healthy hairless rats
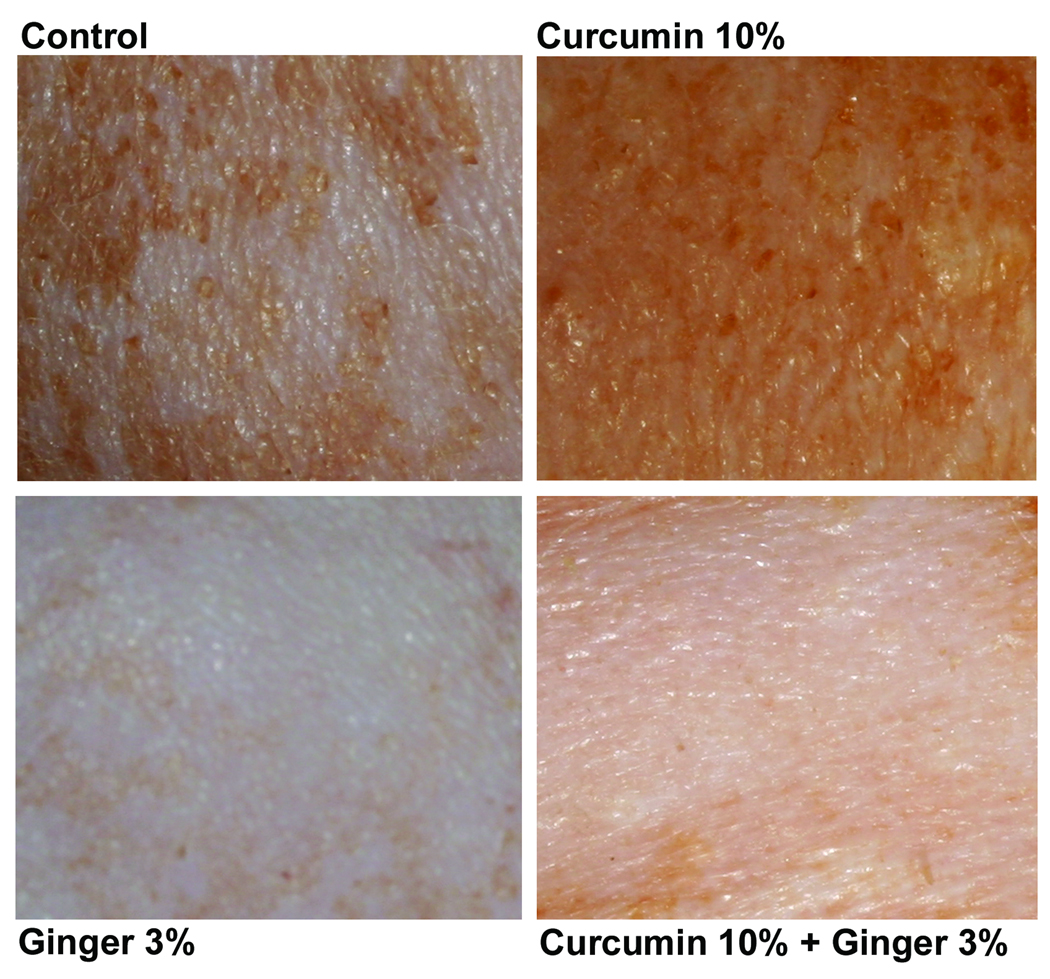
Based on preliminary in vitro experiments in which a wide range of concentrations of both curcumin and the ginger extract were tested (not shown), healthy hairless rats were treated topically once daily for 15 days with solutions containing 10% curcumin, 3% ginger extract or the combination of the two. Control rats were treated with DMSO alone. Animals were monitored closely during the treatment period for visible changes in gross appearance of the skin. Figure 1 demonstrates the appearance of the skin from animals at the end of the 15-day treatment period. Features to note include a consistent reduction in mottling seen with the ginger extract with or without curcumin. The ginger extract-treated skin was slightly pink in tone. The only consistent feature of curcumin-treated skin (with or without the ginger extract) was a slight discoloration of the skin – i.e., the skin had a reddish-yellow tone. No irritation was observed with either curcumin or ginger extract alone or with the combination of the two.
Figure 1.
Gross appearance of hairless rat skin following treatment for 15 days with DMSO, 10% curcumin, 3% ginger extract or the combination of curcumin and ginger extract. DMSO-treated skin has a slightly-mottled appearance (similar to what was seen in control skin). Skin from curcumin-treated rats has a similar appearance but is stained a reddish-yellow. Skin treated with ginger extract has a much more pinkish hue than DMSO-treated skin and there is virtually no mottling. Skin treated with the combination of curcumin and ginger extract has features seen in skin treated with either agent alone. That is, it has lost its mottled appearance but still demonstrates staining from the curcumin.
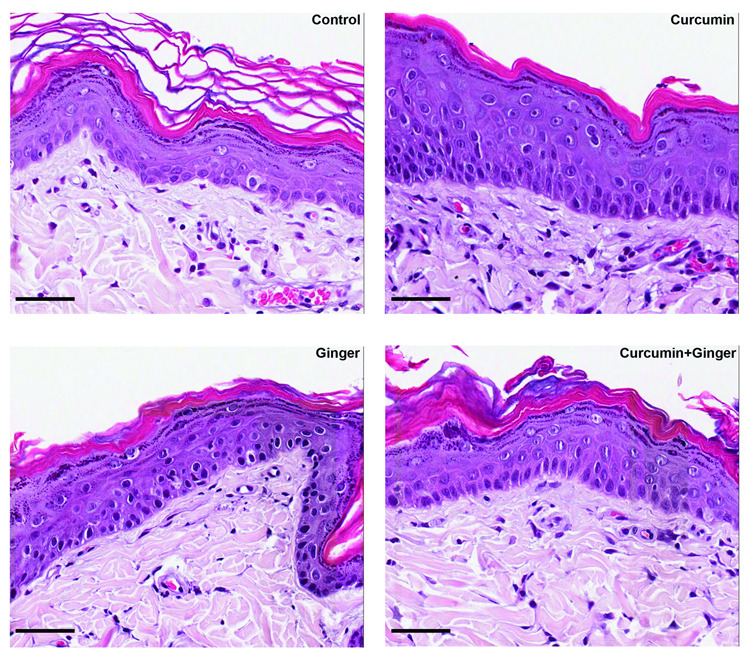
At the end of the treatment period, duplicate biopsies were obtained from the treated site of each animal. One of the biopsies was examined at the light microscopic level after formalin-fixation and staining with hematoxylin and eosin (Figure 2). Overall changes included a slight thickening of the epidermis (seen, primarily, with curcumin) along with a change in basal epithelial cell shape from cuboidal/compressed to columnar. In skin from rats treated with the ginger extract (alone or in combination with curcumin), a consistent feature was an increase in the number of superficial blood vessels. Vessels were counted in duplicate sections and Table 1 summarizes blood vessel counts. As expected, since the treatment period was short, no consistent changes were observed in the structure of the dermal matrix from any of the treatment groups relative to the control.
Figure 2.
Histological appearance of rat skin following treatment for 15 days with DMSO, 10% curcumin, 3% ginger extract or the combination of curcumin and ginger extract. Features to note include a slight thickening of the epidermis in the skin from curcumin-treated rats (with or without ginger) and a more columnar appearance of cells in the basal epithelium. There were no major changes in the appearance of the dermal matrix in any of the treatment groups relative to the DMSO control. In skin from ginger extract-treated animals, (with or without curcumin) there was an increase in the number of blood vessels in the immediate sub-epithelial dermis as compared to DMSO-treated skin. Increased vessel formation was not observed in the skin of rats treated with curcumin alone. Scale bar = 75 µm
Table 1.
Number of blood vessels immediately below the epidermis in the skin of hairless rats following treatment for 15 days with curcumin and/or ginger extract.
| Treatment group | Number of blood vessels |
|---|---|
| DMSO | 9 ± 8 |
| Curcumin | 11 ± 4 |
| Ginger extract | 23 ± 6* |
| Curcumin plus ginger extract | 15 ± 7 |
Values are means and standard deviations based on duplicate histological sections from three or four animals per treatment group. Values were analyzed for statistical significance by ANOVA followed by paired group comparisons.
indicates statistically significant difference from the DMSO group at p<0.05.
The second biopsy was incubated in organ culture for 2 days. At the end of the incubation period, organ culture fluids were collected and analyzed for soluble type I collagen as well as for MMP-2 and MMP-9. Organ culture fluid from skin sites treated with curcumin and from skin sites treated with the combination of curcumin and ginger extract had increased type I collagen and decreased MMP-9 as compared to control skin organ culture fluid (not shown). There was also a decrease in MMP-2 but it was not as substantial as the decrease in MMP-9. Based on these findings, subsequent wounding studies were conducted with 10% curcumin and/or 3% ginger extract.
Topical pretreatment with curcumin and ginger extract improves abrasion wound healing in skin of corticosteroid-treated hairless rats
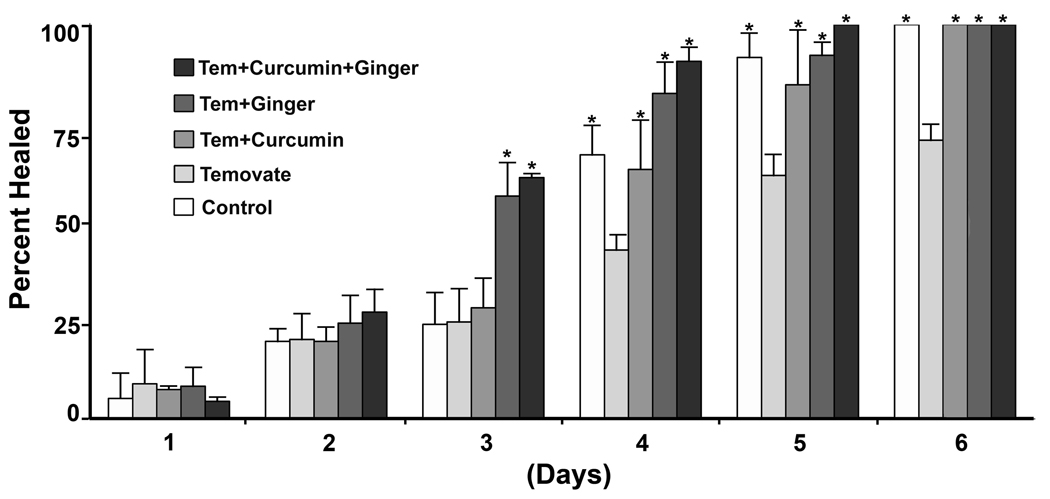
In the next series of experiments, hairless rats were treated with curcumin and ginger extract (alone or in combination) for 21 days. Following this, animals were treated once daily for an additional 15 days with corticosteroid (Temovate). Treatment with the curcumin and ginger extract was continued. Hairless rats that had been treated with vehicle alone during the initial 21-day period were also treated daily with the corticosteroid. These animals continued to receive DMSO as a control. At the end of the treatment phase, abrasion wounds were induced in all animals and healing times were recorded. Three independent experiments with 3 animals in each group were conducted and the pooled data are presented in Figure 3. Wound closure in the corticosteroid-treated animals was slower than in the non-steroid-treated control animals. Wound closure was improved in those steroid-treated rats that also received either curcumin or ginger extract alone as compared to vehicle alone. Rats treated with the combination of curcumin and ginger also demonstrated improved wound healing rates.
Figure 3.
Abrasion wound healing in control hairless rats and hairless rats treated with Temovate plus either DMSO, curcumin, ginger extract or the combination of curcumin and ginger extract. Values represent the percentage of the wound that is closed and scab-free at each time-point. Values are based on 9 animals per group. Differences were evaluated statistically (ANOVA followed by paired group comparisons). * indicates statistically significant difference from the Temovate alone group at p<0.05.
At the time of wound closure, duplicate biopsies were obtained from the center of the initial wound (most recently healed skin). As was done with non-wounded skin, one biopsy was examined histologically and the other placed in organ culture. When examined at the light microscopic level, skin from all groups looked similar. Epidermal hyperplasia was evident and the dermis was characterized by the presence of a provisional matrix separating the recently-healed epidermis from the underlying collagen (not shown).
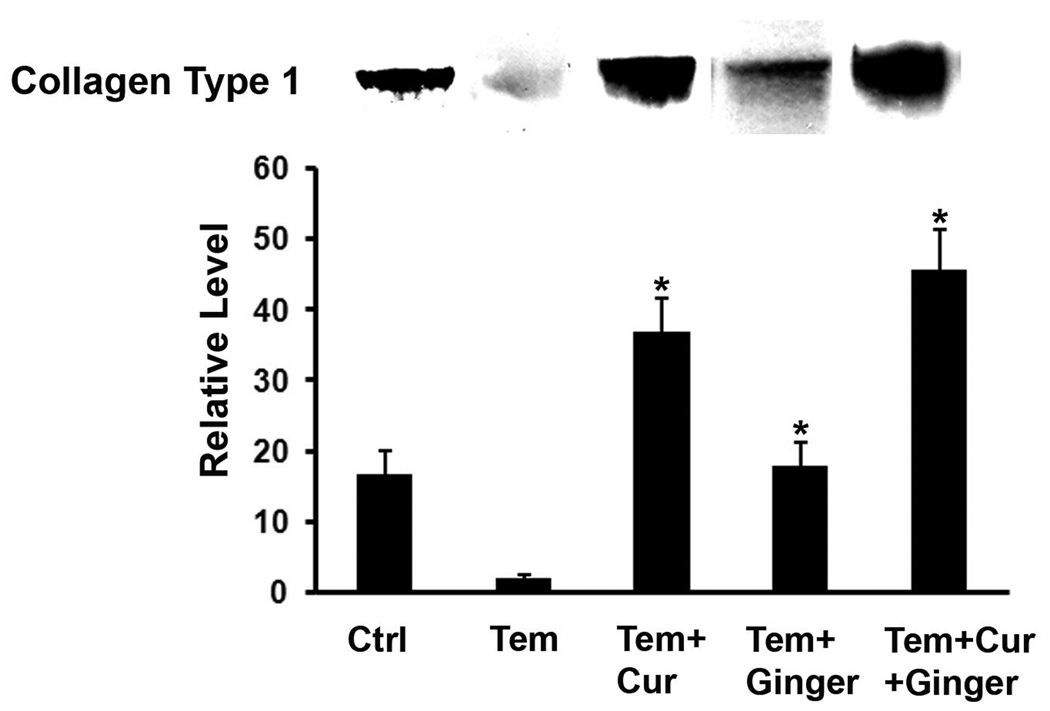
There were differences in organ culture fluid among the treatment groups. As seen in Figure 4, type I collagen was reduced in the culture fluid from skin of rats treated with Temovate as compared to control animals. This is consistent with past results (20) demonstrating steroid-inhibition of collagen production in the skin following topical treatment. Rats treated with curcumin and/or ginger extract prior to corticosteroid treatment (and during steroid treatment) demonstrated elevated levels of type I collagen as compared to rats treated with Temovate and vehicle alone. Interestingly, collagen levels were higher following curcumin treatment (along with Temovate) than were collagen levels in control culture fluids (i.e., from rats not exposed to Temovate). The ginger extract alone also increased type I collagen levels in skin from Temovate-treated rats, but not to the same extent as curcumin alone or curcumin and ginger extract together.
Figure 4.
Soluble collagen (Western blotting) in organ culture fluid of skin from control rats and from rats treated with Temovate plus vehicle, curcumin, ginger extract or curcumin and ginger extract. The biopsy was taken from an area within the initial abrasion wound margin at the time of wound-closure. Values are means and standard deviations based on organ cultures from 9 rats per treatment group. Values were analyzed for statistical significance by ANOVA followed by paired group comparisons. * indicates statistically significant difference from the Temovate plus vehicle group at p<0.05. Insert: Representative western blots of organ culture fluid from each of the treatment groups.
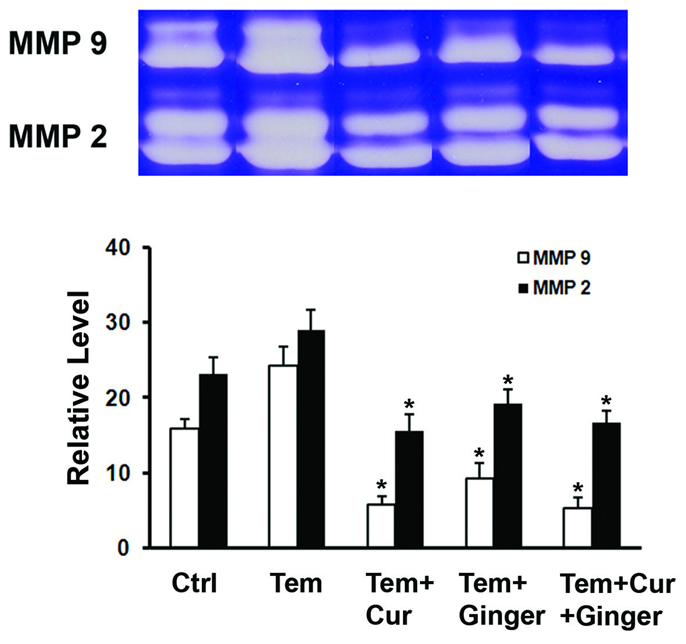
Gelatin zymography data are presented in Figure 5. It can be seen from the figure that levels of both MMP-2 and MMP-9 were elevated in organ culture fluid of skin from Temovate-treated rats relative to levels in control rat skin organ culture. Levels of both enzymes were reduced by treatment with the curcumin-ginger extract. MMP-9 was more sensitive to modulation than was MMP-2. With both enzymes, the decrease was observed equally in the latent and active forms, suggesting that neither agent prevented processing of latent enzyme forms.
Figure 5.
MMP-2 and MMP-9 (gelatin zymography) in organ culture fluid of skin from control rats and from rats treated with Temovate plus vehicle, curcumin, ginger extract or curcumin and ginger extract. The biopsy was taken from an area within the initial abrasion wound margin. Values are means and standard deviations based on organ cultures from 9 rats per treatment group. Values were analyzed for statistical significance by ANOVA followed by paired group comparisons. * indicates statistically significant difference from the Temovate alone group at p<0.05. Insert: Representative gelatin zymograms of organ culture fluid from each of the treatment groups.
DISCUSSION
Superficial wounds that occur in healthy skin are expected to heal without incident. In contrast, wounds in chronically-damaged or atrophic skin heal more slowly and often go on to form non-healing ulcers with devastating consequences (1–11). Multiple factors contribute to impaired wound healing. Past studies suggest that damage to the underlying connective tissue is a major impediment to efficient wound closure. When there is extensive damage to the type I collagen in the dermis (i.e., the major connective tissue element in the dermal matrix), function of the major cell types in the skin is compromised. For example, connective tissue production in the dermis is reduced (40–42) and keratinocyte coverage of the open wound surface is slowed (43). Chronic inflammation and decreased vasculature are other impediments. Similar to what is seen when cells interact with damaged collagen, function of resident skin cells is compromised by the lack of healthy vasculature as well as by chronic inflammation. These impediments to effective wound healing are all interconnected. Efforts to repair damaged skin should take these multiple deficits into consideration.
In the present study, we show that topical pre-treatment with a combination of two natural products – i.e., curcumin and ginger extract – improves healing of subsequently-induced abrasion skin wounds in corticosteroid-treated rats. No irritation was observed in the treated animals at any time during the pretreatment phase or during wounding/wound-healing. How the combination of curcumin and ginger extract functions to improve wound-healing in corticosteroid-damaged skin is not fully understood. The two agents have a variety of effects that could contribute (24–38). Taking past observations into account, along with the findings presented here, we hypothesize that the two agents act through complementary mechanisms. A major contribution of the ginger extract, we suggest, is to increase vascularity and blood flow in the repairing tissue, while the major effect of curcumin is on matrix remodeling. Rats pretreated with ginger extract had a greater number of vessels in the skin than were seen in control rats or rats treated with curcumin alone. In contrast, type I collagen levels were higher in skin from rats pretreated with curcumin (alone or with ginger extract) than in the skin of control rats or rats pretreated with ginger extract. Obviously, additional studies will be needed to substantiate this overall hypothesis. At the present time, we are using human skin in organ culture to help elucidate the mechanisms by which this combination of moieties might work. In the human skin culture system, the ginger extract raises the level of matrix metalloproteinase-1 (MMP-1) – known to be associated with vascular growth into collagen (44) – while curcumin has a strong positive effect on collagen synthesis and suppresses the increase in MMP-1 induced by ginger (manuscript in preparation). These data provide a basis for our suggestion that the two agents have complementary effects. It should be noted that these are not the only potential mechanisms by which the two agents may work. A number of past studies have shown that curcumin and, especially, ginger extract have anti-oxidant properties and broad anti-inflammatory activities (24,26–28,31,33,34).
An important question concerns the active moieties in each of the two botanical preparations. The Indian spice turmeric (Curcumin longa) contains three major curcuminoids, namely, curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Curcumin is the principal curcuminoid in turmeric (30). The curcumin preparation used here is greater than 95% pure. The ginger root (25) is also a source of multiple biologically-active moieties, including 6-gingerol, 8-gingerol, 10-gingerol, 6-paradol, 6-shagoal and cassumunin. 6-Gingerol is the most abundant analogue in the extract used here, and represents approximately 32% of the total extract. At present, we cannot rule out contributions from multiple moieties in the ginger extract. However, in vitro studies have demonstrated that 6-gingerol purified to greater than 95% appears to have the same activities as the crude extract itself (unpublished). Whether the preparation that is ultimately developed makes use of the ginger root extract or one or more of its individual constituents will depend on the outcome of future studies. The fact that both curcumin and ginger are readily available in nature should foster future development efforts. Alternatively, synthetic curcuminoids and synthetic gingerols are also available (30). If it can be shown that the synthetic moieties have wound-healing potential similar to that of the natural compounds, these should also be considered for development. The goal, ultimately, is to have a preparation that is inexpensive to prepare so that individuals at risk for the development of non-healing skin ulcers can utilize it on a long-term basis as a wound preventative.
In summary, while many different approaches have been (and are being) tried to improve healing of wounds when they occur in atrophic or damaged skin, what we propose here is a strategy for improving structure and function in skin prior to injury. The assumption is that if the skin is healthier, it will be less likely to develop chronic wounds after the minor scrapes and bumps that periodically occur. Healthier skin should heal more efficiently when minor injuries occur, and therefore, be less likely to form the chronic, non-healing ulcers that are a major problem for aged individuals, people on chronic corticosteroid use and especially people with diabetes. While our vision is a topical product containing a combination of curcumin and ginger, both agents have been used orally. Thus, it is conceivable that beneficial effects could be attained systemically with these agents provided as a nutritional supplement. Since both agents are GRAS-listed and have a long tradition of use in humans, there is no reason why studies should not be undertaken to determine if delivery through either route provides sufficient skin improvement to warrant full-scale development.
ACKNOWLEDGMENTS
This study was supported in part by National Institutes of Health grants GM77724 and GM80779 from the United States Public Health Service.
REFERENCES
- 1.Strigini L, Ryan T. Wound healing in elderly human skin. Clin Dermatol. 1996;14(2):197–206. doi: 10.1016/0738-081x(95)00155-9. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerentology. 2002;3(6):337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 3.Brem H, Tomic-Canic M, Tarnovskaya A, Ehrlich HP, Baskin-Bey E, Gill K, Carasa M, Weinberger S, Entero H, Vladeck B. Healing of elderly patients with diabetic foot ulcers, venous stasis ulcers, and pressure ulcers. Surg Technol Int. 2003;11:161–167. [PubMed] [Google Scholar]
- 4.Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998;11(6):277–285. [PubMed] [Google Scholar]
- 5.Wicke C, Halliday B, Allen D, Roche NS, Scheuenstuhl H, Spencer MM, Roberts AB, Hunt TK. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135(11):1265–1270. doi: 10.1001/archsurg.135.11.1265. [DOI] [PubMed] [Google Scholar]
- 6.Brand PW. Repetitive stress in the development of diabetic foot ulcers. In: Levin ME, O’Neal LW, editors. The diabetic foot. ed 4. St. Louis: Mosby; 1988. pp. 83–90. [Google Scholar]
- 7.LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984;311(25):1615–1619. doi: 10.1056/NEJM198412203112506. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds ME. Progress in care of the diabetic foot. Lancet. 1999;354(9175):270–272. doi: 10.1016/s0140-6736(99)90012-0. [DOI] [PubMed] [Google Scholar]
- 9.Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg. 1998;176(2A Suppl):39S–47S. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 10.Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A. Effect of age on wound healing in healthy human beings. Surgery. 1992;112(2):293–297. [PubMed] [Google Scholar]
- 11.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329(8):530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 13.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 14.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1463–1465. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 15.Frosch PJ, Czarnetzki BM. Effect of retinoids on wound healing in diabetic rats. Arch Dermatol Res. 1989;281(6):424–426. doi: 10.1007/BF00455329. [DOI] [PubMed] [Google Scholar]
- 16.Popp C, Kligman AM, Stoudemayer TJ. Pretreatment of photoaged forearm skin with topical tretinoin accelerates healing of full-thickness wounds. Br J Dermatol. 1995;132(1):46–53. doi: 10.1111/j.1365-2133.1995.tb08623.x. [DOI] [PubMed] [Google Scholar]
- 17.Otley CC, Gayner SM, Ahmed I, Moore EJ, Roenigk RK, Sherris DA. Preoperative and postoperative topical tretinoin on high-tension excisional wounds and full-thickness skin grafts in a porcine model: a pilot study. Dermatol Surg. 1999;25(9):716–721. doi: 10.1046/j.1524-4725.1999.99005.x. [DOI] [PubMed] [Google Scholar]
- 18.Kitano Y, Yoshimura K, Uchida G, Sato K, Harii K. Pretreatment with topical all-trans-retinoic acid is beneficial for wound healing in genetically diabetic mice. Arch Dermatol Res. 2001;293(10):515–521. doi: 10.1007/pl00007466. [DOI] [PubMed] [Google Scholar]
- 19.Lateef H, Abatan OI, Aslam MN, Stevens MJ, Varani J. Topical pretreatment of diabetic rats with all-trans retinoic acid increases healing of subsequently induced abrasion wounds. Diabetes. 2005;54(3):855–861. doi: 10.2337/diabetes.54.3.855. [DOI] [PubMed] [Google Scholar]
- 20.Warner RL, Bhagavathula N, Nerusu K, Hanosh A, McClintock SD, Naik MK, Johnson KJ, Ginsburg I, Varani J. MDI 301, a nonirritating retinoid, improves abrasion wound healing in damaged/atrophic skin. Wound Repair Regen. 2008;16(1):117–124. doi: 10.1111/j.1524-475X.2007.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths CE, Kang S, Ellis CN, Kim KJ, Finkel LJ, Ortiz-Ferrer LC, White GM, Hamilton TA, Voorhees JJ. Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin creams. Arch Dermatol. 1995;131(9):1037–1044. [PubMed] [Google Scholar]
- 22.Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, Tavakkol A, Yi JY, Griffiths CE, Elder JT, Voorhees JJ. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid-binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105(4):549–556. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- 23.Phillips TJ, Gottlieb AB, Leyden JJ, Lowe NJ, Lew-Kaya DA, Sefton J, Walker PS, Gibson JR. Efficacy of 0.1% tazarotene cream for the treatment of photodamage: a 12-month multicenter, randomized trial. Arch Dermatol. 2002;138(11):1486–1493. doi: 10.1001/archderm.138.11.1486. [DOI] [PubMed] [Google Scholar]
- 24.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, Surh YJ. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Ongogene. 2005;24(15):2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 25.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 26.Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96(1–2):207–210. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Kota N, Krishna P, Polasa K. Alterations in antioxidant status of rats following intake of ginger through diet. Food Chem. 2008;106:991–996. [Google Scholar]
- 28.Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14(2–3):123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290(1–2):87–96. doi: 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- 30.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 31.Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51(5):927–931. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J Biol Chem. 2005;280(10):9409–9415. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- 33.Mani H, Sidhu GS, Kumari R, Gaddipati JP, Seth P, Maheshwari RK. Curcumin differentially regulates TGF-beta1, its receptors and nitric oxide synthesis during impaired wound healing. Biofactors. 2002;16(1–2):29–43. doi: 10.1002/biof.5520160104. [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee Turmeric and curcumin: biological actions and medicinal applications. Current Sci. 2004;87(1):44–53. [Google Scholar]
- 35.Horie S, Yamamoto H, Michael GJ, Uchida M, Belai A, Watanabe K, Priestley JV, Murayama T. Protective role of vanilloid receptor type 1 in HCl-induced gastric mucosal lesions in rats. Scand J Gastroenterol. 2004;39(4):303–312. doi: 10.1080/00365520310008647. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999;7(5):362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 37.Singer AJ, McClain SA, Romanov A, Rooney J, Zimmerman T. Curcumin reduces burn progression in rats. Acad Emerg Med. 2007;14(12):1125–1129. doi: 10.1197/j.aem.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemibody gamma-irradiation. Plast Reconstr Surg. 2005;115(2):515–528. doi: 10.1097/01.prs.0000148372.75342.d9. [DOI] [PubMed] [Google Scholar]
- 39.Varani J, Perone P, Merfert MG, Moon SE, Larkin D, Stevens MJ. All-trans retinoic acid improves structure and function of diabetic rat skin in organ culture. Diabetes. 2002;51(12):3510–3516. doi: 10.2337/diabetes.51.12.3510. [DOI] [PubMed] [Google Scholar]
- 40.Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varani J, Schuger L, Dame MK, Leonard C, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Invest Dermatol. 2004;122(6):1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- 42.Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168(6):1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137(6):1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varani J, Perone P, Warner RL, Dame MK, Kang S, Fisher GJ, Voorhees JJ. Vascular tube formation on matrix metalloproteinase-1 - damaged collagen. Brit J Cancer. 2008;98:1646–1652. doi: 10.1038/sj.bjc.6604357. [DOI] [PMC free article] [PubMed] [Google Scholar]