Abstract
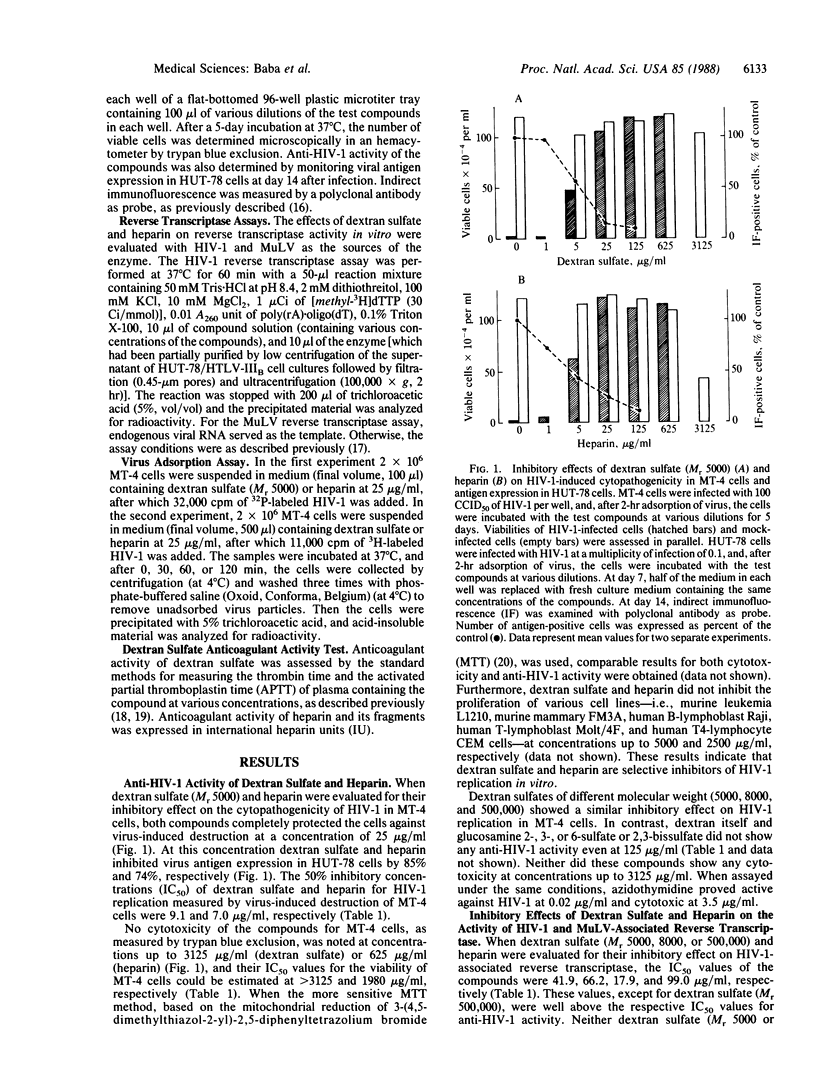
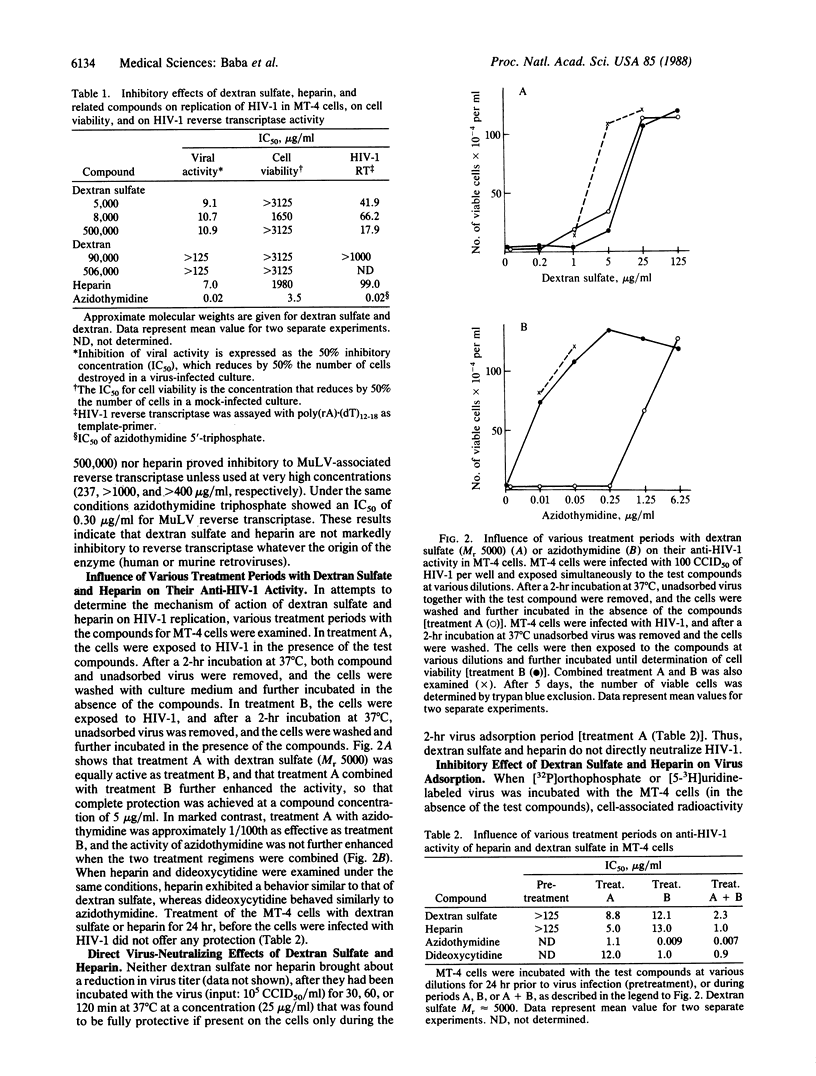
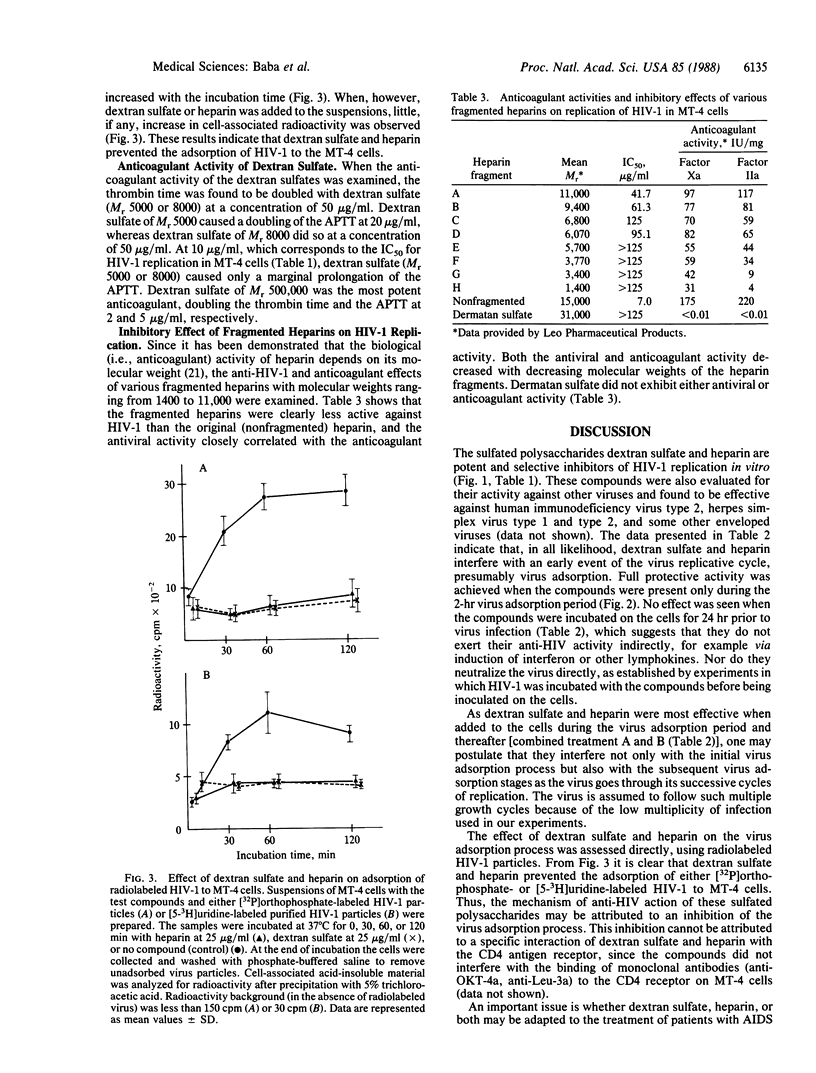
The sulfated polysaccharides dextran sulfate and heparin have proved to be potent and selective inhibitors of human immunodeficiency virus type 1 (HIV-1) in vitro. Dextran sulfate (Mr 5000) and heparin (Mr 15,000) completely protected MT-4 cells against HIV-1-induced cytopathogenicity at a concentration of 25 micrograms/ml. Their 50% inhibitory concentrations were 9.1 micrograms/ml (dextran sulfate) and 7.0 micrograms/ml (heparin), respectively. No toxicity for the host cells was observed with these compounds at a concentration of 625 micrograms/ml. The anti-HIV-1 activity of heparins of various molecular weights correlated well with their anticoagulant activity. On the other hand, with dextran sulfates of low molecular weight (5000, 8000) a significant inhibitory effect on HIV-1 was achieved at a concentration that was not markedly inhibitory to the blood coagulation process. Dextran sulfate and heparin were not inhibitory to HIV-1 reverse transcriptase unless they were used at concentrations in excess of those that inhibited HIV-1 replication. They were highly effective against HIV-1 replication even when present only during the 2-hr virus adsorption period. Studies using radiolabeled HIV-1 virions indicated that dextran sulfate and heparin inhibit virus adsorption to the host cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balzarini J., De Clercq E., Torrence P. F., Mertes M. P., Park J. S., Schmidt C. L., Shugar D., Barr P. J., Jones A. S., Verhelst G. Role of thymidine kinase in the inhibitory activity of 5-substituted-2'-deoxyuridines on the growth of human and murine tumor cell lines. Biochem Pharmacol. 1982 Mar 15;31(6):1089–1095. doi: 10.1016/0006-2952(82)90347-1. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Mitsuya H., De Clercq E., Broder S. Aurintricarboxylic acid and Evans Blue represent two different classes of anionic compounds which selectively inhibit the cytopathogenicity of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. Biochem Biophys Res Commun. 1986 Apr 14;136(1):64–71. doi: 10.1016/0006-291x(86)90877-6. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Broder S., Yarchoan R., Collins J. M., Lane H. C., Markham P. D., Klecker R. W., Redfield R. R., Mitsuya H., Hoth D. F., Gelmann E. Effects of suramin on HTLV-III/LAV infection presenting as Kaposi's sarcoma or AIDS-related complex: clinical pharmacology and suppression of virus replication in vivo. Lancet. 1985 Sep 21;2(8456):627–630. doi: 10.1016/s0140-6736(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Dawes J., Bara L., Billaud E., Samama M. Relationship between biological activity and concentration of a low-molecular-weight heparin (PK 10169) and unfractionated heparin after intravenous and subcutaneous administration. Haemostasis. 1986;16(2):116–122. doi: 10.1159/000215281. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Chemotherapeutic approaches to the treatment of the acquired immune deficiency syndrome (AIDS). J Med Chem. 1986 Sep;29(9):1561–1569. doi: 10.1021/jm00159a001. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett. 1979 Nov;8(1):9–22. doi: 10.1016/0304-3835(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Folkman J., Langer R., Linhardt R. J., Haudenschild C., Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983 Aug 19;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Fyfe J. A., St Clair M. H., Weinhold K., Rideout J. L., Freeman G. A., Lehrman S. N., Bolognesi D. P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Gelmann E. P., Robert-Guroff M., Richardson E., Kalyanaraman V. S., Mann D., Sidhu G. D., Stahl R. E., Zolla-Pazner S. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Owen W. G., Collen D. Involvement of heparin chain length in the heparin-catalyzed inhibition of thrombin by antithrombin III. J Biol Chem. 1984 May 10;259(9):5670–5677. [PubMed] [Google Scholar]
- Ito M., Baba M., Sato A., Pauwels R., De Clercq E., Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Res. 1987 Jul;7(6):361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Cossman J., Megson M., Reitz M. S., Jr, Broder S. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I). Science. 1984 Sep 28;225(4669):1484–1486. doi: 10.1126/science.6206569. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Looney D. J., Kuno S., Ueno R., Wong-Staal F., Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988 Apr 29;240(4852):646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- Naggi A., Torri G., Casu B., Pangrazzi J., Abbadini M., Zametta M., Donati M. B., Lansen J., Maffrand J. P. "Supersulfated" heparin fragments, a new type of low-molecular weight heparin. Physico-chemical and pharmacological properties. Biochem Pharmacol. 1987 Jun 15;36(12):1895–1900. doi: 10.1016/0006-2952(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., De Clercq E., Desmyter J., Balzarini J., Goubau P., Herdewijn P., Vanderhaeghe H., Vandeputte M. Sensitive and rapid assay on MT-4 cells for detection of antiviral compounds against the AIDS virus. J Virol Methods. 1987 Jun;16(3):171–185. doi: 10.1016/0166-0934(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Refsum N., Collen D., Godal H. C., Janson T., Mannucci P. M., Nilsson I. M., Gilhuus-Moe C. C. Sensitivity and precision of activated partial thromboplastin time (APTT) methods. A multicenter study. Scand J Haematol. 1978 Jan;20(1):89–95. doi: 10.1111/j.1600-0609.1978.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Fischl M. A., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Hirsch M. S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- SASAKI S., TAKEMOTO T., OKA S. TOXICITY OF HEPARINOIDS, WITH SPECIAL REFERENCE TO THE PRECIPITATION OF FIBRINOGEN. Thromb Diath Haemorrh. 1964 Oct 15;12:232–261. [PubMed] [Google Scholar]
- Ueno R., Kuno S. Dextran sulphate, a potent anti-HIV agent in vitro having synergism with zidovudine. Lancet. 1987 Jun 13;1(8546):1379–1379. doi: 10.1016/s0140-6736(87)90681-7. [DOI] [PubMed] [Google Scholar]
- VERMYLEN C., VERSTRAETE M. Antithrombin V: Critical evaluation of its assessment and properties. Thromb Diath Haemorrh. 1960 Dec 15;5:267–284. [PubMed] [Google Scholar]
- Yarchoan R., Klecker R. W., Weinhold K. J., Markham P. D., Lyerly H. K., Durack D. T., Gelmann E., Lehrman S. N., Blum R. M., Barry D. W. Administration of 3'-azido-3'-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex. Lancet. 1986 Mar 15;1(8481):575–580. doi: 10.1016/s0140-6736(86)92808-4. [DOI] [PubMed] [Google Scholar]


