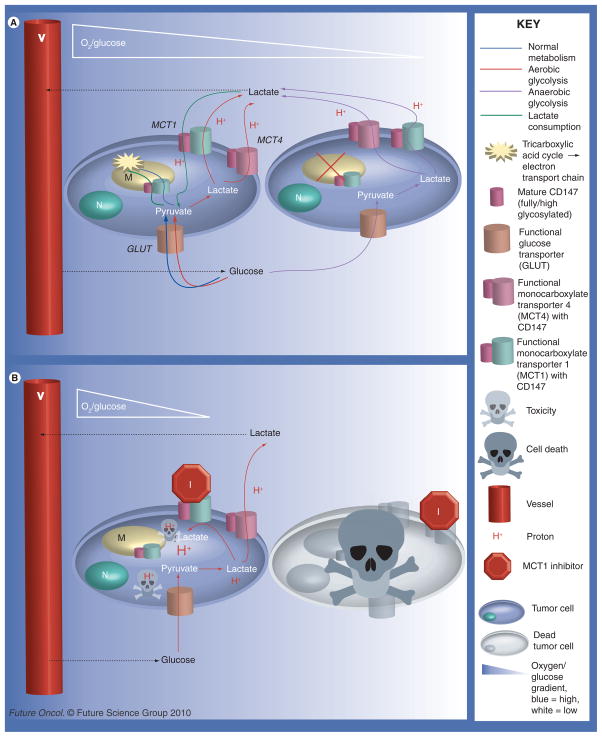
Figure 3. Potential metabolic pathways between tumor cells (see left).
Cells close to vessels will have the advantage of high/adequate concentrations of oxygen and nutrients, such as glucose. Cells farther from vessels will experience varying degrees of hypoxia and starvation. (A) The metabolic pathways possible between cancer cells without MCT1 inhibition when the aerobic cells are able to consume/utilize lactate. Shown here is the Pasteur effect (anaerobic glycolysis) in the hypoxic cells farther from vessels. The well-oxygenated cells close to vessels may undergo healthy oxidative phosphorylation, possibly the Warburg effect (aerobic glycolysis), or lactate utilization. (B) Illustrates the consequences of MCT1 inhibition on cell-to-cell metabolism and intracellular pH. MCT4, having a high Km, is unlikely to take up lactate unless there is a very high extracellular concentration of lactate. Excluded from this diagram are pH regulators other than MCTs, such as Na+/H+ exchanger (NHE1), which would serve to remove some of the H+ from the cell. MCT1 inhibition can lead to cell death by two different means: the hypoxic cells are starved since cells close to vessels are forced to only take up glucose since the ability of lactate consumption is blocked, as seen by comparing (A) to (B) or the decrease in intracellular pH leads to toxicity, indicated by the protons represented in the diagram.