Abstract
Rationale
Acute re-exposure to cocaine or drug cues associated with cocaine use can elicit drug craving and relapse. Neuroimaging studies have begun to define neurobiological substrates underlying the acute effects of cocaine or cocaine cues in cocaine-dependent subjects.
Objective
The present study was the first to use functional brain imaging to document acute cocaine-induced changes in brain activity during active drug use in nonhuman primates.
Materials and methods
Positron emission tomography imaging with O15-labeled water was used to measure drug-induced changes in cerebral blood flow. The acute effects of cocaine administered noncontingently were characterized in four drug-naïve rhesus monkeys. The same subjects were trained to self-administer cocaine under a fixed ratio schedule during image acquisition. Subsequently, three subjects with an extensive history of cocaine use were trained to self-administer cocaine under a secondorder schedule. The same subjects also underwent extinction sessions during which saline was substituted for cocaine under the second-order schedule.
Results
Noncontingent administration of cocaine in drugnaïve subjects induced robust activation of prefrontal cortex localized primarily to the dorsolateral regions. In contrast, the pattern of brain activation induced by self-administered cocaine differed qualitatively and included anterior cingulate cortex. Moreover, drug-associated stimuli during extinction also induced robust activation of prefrontal cortex.
Conclusions
The effects of cocaine and associated cues extend beyond the limbic system to engage brain areas involved in cognitive processes. The identification of neural circuits underlying the direct pharmacological and conditioned stimulus effects of cocaine may be highly relevant toward efforts to develop treatments for cocaine addiction.
Keywords: Cocaine, Cerebral blood flow, PET imaging, Self-administration, Extinction, Nonhuman primates
Introduction
A major difficulty in treating cocaine addiction is the high rate of relapse after periods of drug abstinence (Jaffe 1990; Leshner 1997; Paulus et al. 2005). Acute re-exposure to cocaine or drug cues associated with cocaine use can elicit drug craving and relapse (Jaffe et al. 1989;O’Brien et al. 1992, 1998; Childress et al. 1993). Studies employing neuroimaging techniques have begun to define neurobiological substrates underlying the acute effects of cocaine or exposure to cocaine cues in cocaine-dependent subjects. For example, acute cocaine administration increased cerebral blood flow mainly in the frontal and parietal regions (Mathew et al. 1996). A blood oxygen level-dependent functional magnetic resonance imaging (fMRI) study reported dynamic patterns of brain activation following cocaine administration (Breiter et al. 1997). Regions including cingulate and lateral prefrontal cortex showed short duration activations that were correlated with ratings of “rush”. Other regions including the nucleus accumbens showed sustained activation associated with ratings of “craving”. Cocaine also activated mesolimbic and mesocortical regions that receive dopaminergic afferents (Kufahl et al. 2005).
Related studies have documented that specific brain regions activated by cocaine are also activated by drug-associated cues. For example, cocaine users showed increases in cerebral blood flow in the anterior cingulate and amygdala when watching a video containing cocainerelated scenes (Childress et al. 1999). Similarly, when script-guided imagery of autobiographical memories was used to induce cocaine craving, subjects showed significant increases in cerebral blood flow in the anterior cingulate and amygdala (Kilts et al. 2001). Additional studies using fMRI have reported activation of anterior and posterior cingulate and dorsolateral prefrontal cortex in response to cocaine-related videos (Maas et al. 1998; Garavan et al. 2000; Wexler et al. 2001). More recently, there is evidence to suggest that the pattern and time course of brain activation in response to cocaine cues may predict treatment outcomes (Kosten et al. 2006).
The capability to conduct parallel neuroimaging studies in nonhuman primates and human subjects provides a powerful translational approach that can link findings from human and animal research. A significant advantage of nonhuman primate models is the use of initially drug-naïve subjects in longitudinal designs to characterize within-subject changes in aspects of the neurobiology associated with chronic drug use. The present study was the first to use functional brain imaging to document acute cocaine-induced changes in brain activity during active drug use in nonhuman primates. Positron emission tomography (PET) imaging with O15-labeled water was used to measure drug-induced changes in cerebral blood flow. First, the acute effects of cocaine administered noncontin-gently were characterized in four drug-naïve rhesus monkeys. Secondly, the same four subjects were trained to self-administer cocaine under a simple fixed ratio (FR) schedule during image acquisition. Thirdly, three subjects with an extensive history of cocaine use were trained to self-administer cocaine under a complex, second-order schedule. Finally, the effectiveness of drug-related stimuli to induce changes in cerebral blood flow was characterized during extinction conditions in the same three subjects. Collectively, the results highlight the important role of drug-paired environmental stimuli in modulating brain activity and provide a unique nonhuman model to evaluate the effectiveness of pharmacological therapies to treat cocaine addiction.
Materials and methods
Subjects
Three female (RRg-4, RGi-4, and RLl-4) and two male (RMk-3 and RDp-3) adult rhesus monkeys (Macaca mulatta) weighing 7.2–11.5 kg served as subjects. Each subject was housed individually and fed Purina monkey chow (Ralston Purina, St. Louis, MO, USA), fruits, and vegetables. Water was continuously available. Animal care procedures strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Surgery
Each subject was prepared surgically with a chronic indwelling venous catheter under sterile surgical conditions using a technique described previously (Howell and Wilcox 2001). Preoperative antibiotics (Rocephin (ceftriaxone, 25 mg/kg)) were given on the day of the surgery to prevent infection. A silicone catheter (0.65 mm i.d., 1.75 mm o.d.; Access Technologies, Skokie, IL, USA) was implanted under a combination of Telazol (tiletamine HCl and zolazepam HCl, 4.0 mg/kg) and isoflurane anesthesia using aseptic techniques. The proximal end of the catheter terminated in the vena cava above the right atrium, and the distal end was routed under the skin and attached to a subcutaneous vascular access port (Access Technologies) located in the center of the lower back. After surgery, the subject was returned to its home cage and received Banamine (flunixin meglumine, 1.0 mg/kg) every 6 for 24 h postoperatively to reduce potential pain and discomfort associated with surgery. Catheters were flushed daily with 1.0 ml of heparinized (100 U/ml) saline to maintain patency.
Drug
Cocaine HCl (National Institute on Drug Abuse) was dissolved in 0.9% saline, and doses were determined as salts.
Apparatus
Due to the importance of position and alignment factors in imaging studies, specific attention was devoted to the development of an effective and comfortable head restraint device to be used in the imaging of conscious monkeys (Howell et al. 2001). The restraint device was designed to attach to a Primate Products chair and facilitate frequent immobilization. Subjects received extensive behavioral training prior to neuroimaging in order to ensure the comfort of the subjects and minimize potential stress associated with the imaging protocols. The terminal phase of training involved immobilization and transfer from the Yerkes Center to the Emory PET Center on a weekly basis. Objective measures of plasma cortisol levels supported the behavioral observations that the immobilization and neuroimaging protocols were well tolerated and failed to induce significant stress (Howell et al. 2001). For behavioral studies, a response lever was mounted on the right arm of the restraint device and required minimal motion by the subject’s hand to activate the microswitch and register a response. Located in front of the head-restraint device at eye level were red and white stimulus lights. Once the monkey was seated in the chair, a Huber needle (Access Technologies) was inserted into the venous access port. The polyvinyl chloride tubing attached to the Huber needle was connected to a motor-driven syringe (Coulbourn Instruments, Allentown, PA, USA) containing the drug solution. A volume of 2.0 ml/infusion was delivered over 7 s. An IBM compatible computer controlled experimental events and recorded data. PET imaging was performed on a Siemens 951 scanner which can image an entire monkey brain with a 10-cm axial field of view.
Behavioral procedures
The study comprised three distinct phases. In the first phase, the acute effects of cocaine (1.0 mg/kg) were determined in four drug-naïve subjects (RRg-4, RGi-4, RMk-3, and RDp-3). Drug administration was not contingent on a behavioral response by the subjects (noncontingent drug administration). The effects of cocaine were determined twice, and saline (control) was determined three times on separate occasions in all subjects. In the second phase, the same four subjects were trained to lever press under a simple FR20 response schedule of i.v. cocaine delivery (FR self-administration). When the daily session began, the red stimulus light in front of the helmet was illuminated and indicated that cocaine was available. Completion of 20 responses in the presence of the red light resulted in the delivery of a single i.v. dose of cocaine (0.33 mg/kg/injection) or saline and changed the stimulus light from red to white for 15 s. A 60-s time-out period followed the white light presentation, and lever presses had no scheduled consequences. All stimulus lights were extinguished during the 60-s time-out. The behavioral protocol comprised three consecutive FR20 components, each followed by a 60-s time-out. Hence, the total drug dose administered during a session (1.0 mg/kg) was matched to the single bolus dose administered in the first phase of the study. The effects of cocaine and saline (extinction) were both determined twice on separate occasions in all subjects. In the third phase, three subjects (RRg-4, RGi-4, and RLl-4) were trained to lever press under a complex schedule of i.v. cocaine delivery that required the completion of multiple response sequences over consecutive 10-min intervals (second-order self-administration). During the 10-min intervals, completion of each FR20 changed the stimulus light from red to white for 2 s The first FR20 completed after the10-min interval had elapsed resulted in the delivery of a single i.v. dose of cocaine (0.33 mg/kg/injection) or saline and changed the stimulus light from red to white for 15 s. The behavioral protocol comprised three consecutive 10-min intervals, each followed by a 60-s time-out. Hence, total drug dose administered during a session (1.0 mg/kg) was matched to the first two phases of the study. Subjects also underwent extinction sessions during which saline was substituted for cocaine under the second-order schedule conditions. The effects of cocaine were determined twice, and saline (extinction) was determined three times on separate occasions in all subjects.
Regional cerebral blood flow determinations
Because awake primates served as subjects and arterial catheters were not used, global and regional subtraction techniques were used to determine relative flow, normalized to global value. Subjects were positioned in the scanner, and a 15-min transmission scan was obtained for attenuation correction prior to the bolus injection of the radiotracer. For each scan, a 10-mCi i.v. injection of O15 water was administered. After the bolus injection, PET data were obtained for 90 s beginning when activity was first noted in the brain, approximately 10 s after the bolus injection. Two images were acquired prior to drug administration, and the mean value was defined as the session baseline. During self-administration and extinction sessions, the baseline scans were acquired prior to the illumination of the discriminative stimulus and the initiation of responding. Each experimental session also included six sequential i.v. injections of O15 water at 10-min intervals beginning 5 min after the last injection of cocaine or saline. However, drug effects dissipated rapidly and were rarely evident 15–25 min postinjection. Accordingly, all data presented were derived from the first O15 water determination following cocaine or saline administration. The PET images for each subject were registered to MRI. Coregistration of the animal studies used slight modifications of procedures and techniques that are in standard practice for human studies at Emory University. All subsequent water images were aligned to the first. The scans were then added together, and the result was registered to the MRI of each animal using the methods of Woods et al. (1993). Accuracy of coregistration is on the order of 1–2 mm when performed with head phantom studies and in human studies. The MRI images provide excellent anatomical delineation of structures in monkeys.
Data analysis
Image analysis was performed in a manner very similar to previous work (Votaw and Li 1995; Henry et al. 1999; Votaw et al. 1999; Howell et al. 2002). Briefly, all images for each subject were corrected for motion that may have occurred during the acquisition by rigid body registering in 3D all subsequent images to the first using the methods of Woods et al. (1998a). This registration approach was also used to align in 3D the first frame of the functional image set to the anatomical image (Woods et al. 1993). Each anatomical image was then warped into a standard space using the methods described by Woods et. al (1998b). The standard was a mean anatomical MR image created by aligning and averaging eight animal image sets and then manually translating, rotating, and scaling the mean image to roughly fit into the human coordinate system described by Talairach and Tournoux (1988). This standard image consisted of 47 slices of thickness 3 mm; each slice was 121×95 pixels of dimension 1.5×1.5 mm. The registration (functional to anatomical) and warping (anatomical to standard) transforms were combined and applied to each image of the functional data set so that all analyzed images of each subject were in the Talairach frame of reference where the transaxial images are parallel to the anterior commissure–posterior commissure line.
The PET data were analyzed with and without a global normalization. When normalized, each pixel was divided by the average intensity of all pixels within the brain. Only pixels common to all acquired images across all subjects, as determined by the alignment to the standard, were used to calculate the average.
A three-way analysis of variance was applied to the PET studies using subject, repetition, and scan condition as factors. Linear contrasts were computed to test whether the mean pixel intensities were different between control and task states (Neter et al. 1990). The model assumed that there were no interactions between variables. Potential sites of increase in the PET data were evaluated at the overall p<0.05 level of significance using the method of Friston et al. (1994), which considers the spatial extent of the region, and Votaw et al. (1998), which considers the shape of the region as a function of the cutoff threshold. Decreases in regional cerebral blood flow were not considered in the present analyses. Note that the anatomy of the prefrontal cortex is identified in Fig. 1.
Fig. 1.
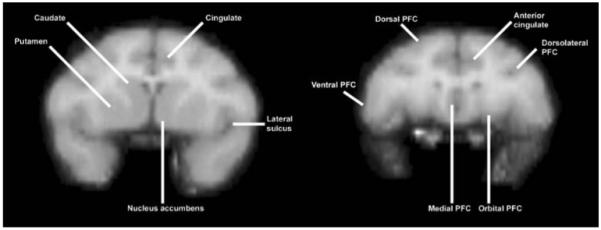
Pictorial representation of the relevant anatomical regions in coronal slices at the level of the striatum (left) and prefrontal cortex (right)
Results
Noncontingent drug administration
Brain activation maps normalized to global flow showed prominent drug-induced activation of prefrontal cortex localized primarily to dorsolateral regions 5 min postinjection (Fig. 2). As reported previously (Howell et al. 2002), drug effects on regional cerebral blood flow were transient and diminished markedly by 25 min postinjection (data not shown). Cocaine also induced a significant increase in whole brain blood flow (Table 1).
Fig. 2.
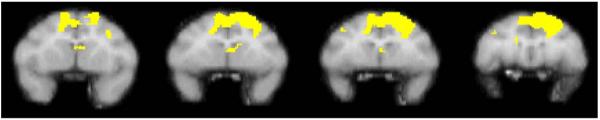
The figure shows a series of sequential coronal slices depicting voxels meeting statistical significance in a group of four monkeys following noncontingent administration of cocaine. The comparison condition was noncontingent administration of saline. Data were modified from Howell et al. 2002
Table 1.
Summary of cerebral blood flow effects
| Condition | % Change in whole brain blood flowa | Primary region of activation |
|---|---|---|
| Noncontingent cocaine | 8.7±4.0* | Dorsolateral prefrontal cortex |
| Cocaine self-administration (fixed ratio) | 1.9±9.3 | Anterior cingulate |
| Cocaine self-administration (second order) | −6.5±4.4 | NA |
| Extinction (second order) | 3.0±3.5 | Dorsomedial prefrontal cortex |
Data were compared to the average of the first two blood flow determinations obtained prior to drug or saline administration
p<0.05 (significant change from baseline values)
Simple fixed ratio self-administration
All subjects learned to self-administer cocaine reliably under the fixed ratio schedule of i.v. drug delivery while positioned in the PET scanner. Daily sessions were conducted in the restraint device at the Yerkes Center, and actual scan acquisition occurred once or twice per month at the Emory PET Center in individual subjects. During experimental sessions, subjects reliably received all three scheduled drug injections. When saline was substituted for cocaine unpredictably under extinction conditions, subjects still reliably received all scheduled injections. Extinction conditions were very infrequent, and the session duration was relatively brief so self-administration behavior remained robust even in the absence of cocaine delivery. It typically required 4–7 min for subjects to complete the behavioral task and receive all scheduled injections. Stimulus control of the learned behavior was evident by rapid initiation of responding in the presence of the red stimulus light and an absence of responding when the red stimulus light was terminated. Brain activation maps normalized to global flow showed prominent cocaine-induced activation localized primarily to the medial region corresponding to the anterior cingulate (Fig. 3). As observed for noncontingent cocaine administration, drug effects on regional cerebral blood flow were transient and diminished markedly by 25 min postinjection (data not shown). Cocaine did not induce a significant change in whole brain blood flow (Table 1).
Fig. 3.
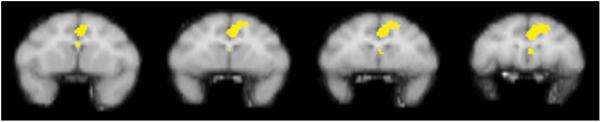
The figure shows a series of sequential coronal slices depicting voxels meeting statistical significance in a group of four monkeys following self-administration of cocaine under an FR schedule. The comparison condition was extinction during which saline was substituted for cocaine. Data were derived from the same four rhesus monkeys described in Fig. 2
Complex second-order self-administration
All subjects learned to self-administer cocaine reliably under the second-order schedule of i.v. drug delivery while positioned in the PET scanner. Due to the demanding requirements of the reinforcement schedule and the longer session duration, behavioral training occurred over a 9–13-month period before the initiation of PET experiments. Drug intake during the period of behavioral training ranged from 82 to 128 mg/kg. During experimental sessions, subjects reliably received all three scheduled injections of cocaine or saline. Stimulus control of the learned behavior was evident by rapid initiation of responding in the presence of the red stimulus light and an absence of responding when the red stimulus light was terminated. The pattern of responding was also typical of performance maintained by second-order schedules with identical parameters (Howell and Wilcox 2001). Once responding was initiated, lever pressing was rapid and sustained until the FR20 requirement was completed and the stimulus light changed from red to white for 2 s. There was generally a pause in lever pressing after completion of each FR20 requirement, followed by a high, steady rate of responding until the subsequent FR20 requirement was completed. Average session response rates ranged from 0.23 to 0.73 responses/s (414–1,918 responses per session). Interestingly, in contrast to the results obtained under the simple FR schedule, brain activation maps normalized to global flow during the second-order condition did not reveal drug-induced activation of any brain region when compared to extinction conditions (Fig. 4). Cocaine did not induce a significant change in whole brain blood flow (Table 1). However, when the statistical comparison was between extinction conditions and noncontingent saline administration, brain activation maps normalized to global flow showed robust activation of dorsomedial prefrontal cortex (Fig. 5). Hence, there was a lack of direct pharmacological effect induced by cocaine, yet there was robust prefrontal activation associated with extinction conditions under the second-order schedule. The effects associated with extinction conditions were transient and diminished markedly by 25 min postinjection (data not shown). Extinction conditions did not induce a significant change in whole brain blood flow (Table 1).
Fig. 4.
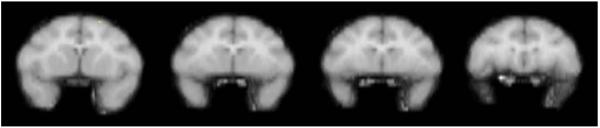
The figure shows a series of sequential coronal slices depicting voxels meeting statistical significance in a group of three monkeys following self-administration of cocaine under a second-order schedule. The comparison condition was extinction during which saline was substituted for cocaine
Fig. 5.
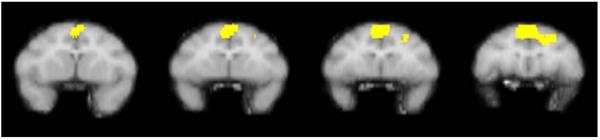
The figure shows a series of sequential coronal slices depicting voxels meeting statistical significance in a group of three monkeys following extinction conditions under a second-order schedule. The comparison condition was noncontingent saline administration. Data were derived from the same three rhesus monkeys described in Fig. 4
Discussion
The present study is the first to use functional brain imaging to document acute cocaine-induced changes in brain activity during active drug use in nonhuman primates. Initial experiments characterized the acute effects of cocaine administered noncontingently in drug-naïve rhesus monkeys. Repeated baseline determinations of cerebral blood flow prior to drug administration were reliable, and brain activation maps normalized to global flow showed prominent cocaine-induced activation of prefrontal cortex localized primarily to the dorsolateral regions. Subsequent experiments characterized the effects of cocaine during active drug self-administration protocols in the same subjects. They were trained initially to respond for intravenous injections of cocaine under a fixed ratio 20 schedule in the presence of a red light, and the stimulus lights changed to white during drug infusion. Appropriate stimulus control of self-administration behavior was established, as evidenced by a lack of responding in the absence of the red light. Compared to noncontingent cocaine administration, the pattern of brain activation induced by self-administered cocaine during the simple fixed ratio schedule differed qualitatively. The area of major activation included anterior cingulate cortex, a region associated with the extended limbic system. A final series of experiments using a complex second-order schedule determined the pattern of brain activation induced by drug-associated stimuli in the absence of cocaine. When the effects of drug-associated stimuli were determined during extinction, there were marked increases in regional cerebral blood flow in the dorsomedial prefrontal cortex, indicating robust cortical activation. Collectively, the results document qualitative differences in the pattern of brain activation induced by cocaine during contingent versus noncontingent drug administration. Moreover, drug-associated stimuli can induce robust activation of prefrontal cortex in subjects with a complex history of drug use. The brain activation induced by cocaine and drug-associated stimuli provides a conceptual framework for characterizing the effects of potential medications on brain activity in awake, behaving monkeys.
The differences observed in the pattern of brain activation following noncontingent versus self-administered cocaine is consistent with a growing literature reporting both quantitative and qualitative differences in the response to cocaine depending on whether the drug is administered passively or self-administered. For example, the presence or absence of response dependency can significantly alter the lethal effects of cocaine in rats (Dworkin et al. 1995). Similarly, self-administered cocaine leads to greater increases in extracellular dopamine in the nucleus accumbens of rats compared to response-independent drug administration (Hemby et al. 1997). Of particular relevance to the present study, the brain metabolic effects of self-administered cocaine in rhesus monkeys (Porrino et al. 2002) differed qualitatively from results obtained in previous experiments utilizing noncontingent drug administration in drug-naïve monkeys (Lyons et al. 1996). Specifically, cocaine self-administration induced a more restricted distribution of changes in functional activity in the medial and orbital prefrontal cortex. Another notable difference was the elevation in cerebral metabolic rates within the dorsolateral and dorsomedial prefrontal cortex of the self-administering animals. In the present study, the history of drug intake also may have contributed to the differences observed following noncontingent versus contingent drug administration. The effects of noncontingent cocaine administration were determined in drug-naïve subjects, whereas self-administration training by necessity required a more extended drug history. Significant differences have been reported in the brain metabolic effects of self-administered cocaine in the striatum of rhesus monkeys based on duration of drug exposure (Porrino et al. 2004). Specifically, in the initial phases of cocaine exposure, self-administration significantly decreased activity in the ventral striatum. In contrast, extended exposure lead to more intense and widespread metabolic effects that included most aspects of the caudate and putamen.
Second-order schedules of drug self-administration allow the investigation of more complex behavioral sequences than do simple schedules and accordingly may better reflect the human condition (Katz and Goldberg 1991; Schindler et al. 2002). Importantly, brief stimulus presentations are critical to the acquisition and maintenance of responding, providing a reliable method to evaluate the effects of conditioned stimuli on drug self-administration behavior. The present study utilized a second-order schedule of cocaine self-administration to characterize the effects of conditioned stimuli on cerebral blood flow under extinction conditions and demonstrated robust activation of prefrontal cortex in the absence of pharmacological effects. It should be noted, however, that the lack of direct pharmacological effects may have been due to the spacing of drug injections over a 30-min period, thereby leading to modest blood levels at the time of scan acquisition. The results obtained under extinction conditions are consistent with studies reporting conditioned responses to drug-associated environmental stimuli in humans. It is well documented that cocaine cues can effectively elicit physiological responses and self-reports of cocaine craving and withdrawal (Ehrman et al. 1992). One potential mechanism is cue-induced dopamine release in the dorsal striatum (Volkow et al. 2006a). Others have reported conditioned dopamine release in the ventral striatum in response to amphetamine cues (Boileau et al. 2007). Interestingly, oral methylphenidate administration in cocaine abusers significantly increased dopamine in the striatum as measured by displacement of C11 raclopride but failed to induce craving unless subjects were concomitantly exposed to cocaine cues (Volkow et al. 2008). Similarly, drug-associated cues have been shown to modulate the brain metabolic effects of stimulants in cocaine abusers. In one study, the brain metabolic effects of methylphenidate were enhanced in cocaine abusers when methylphenidate was administered in the presence of methylphenidate-associated cues (Volkow et al. 2003). Drug-induced increases in self reports of “high” were also greater when subjects received methylphenidate in the presence of methylphenidate-associated cues, and self-report measures were significantly correlated with brain metabolic effects. Similar results have been reported for subjects who had minimal experience with stimulant drugs (Volkow et al. 2006b). It is apparent that conditioning and environmental stimuli can modulate the neurochemical, physiological, and behavioral effects of stimulants, having a profound influence on their addictive properties.
Functional brain imaging has begun to define the neural circuitry underlying the acute pharmacological effects of cocaine, conditioned responses to cocaine-cues, and the experience of drug craving in humans. Activation of the anterior cingulate has been observed in response to acute administration of cocaine and related stimulants (Breiter et al. 1997; Volkow et al. 1999) and cocaine-related environmental cues (Maas et al. 1998; Childress et al. 1999; Kilts et al. 2001; Wexler et al. 2001). Moreover, activation of the dorsolateral prefrontal cortex has also been observed in response to cocaine (Kufahl et al. 2005) and cocaine cues (Maas et al. 1998; Grant et al. 1996). These reliable regional effects highlight the important role of an integrated circuitry in the context of cocaine addiction. The anterior cingulate, part of the extended limbic system, is anatomically linked to the prefrontal cortex and nucleus accumbens and serves diverse functions including the integration of mood and cognition (Vogt et al. 1992; Devinsky et al. 1995). The dorsolateral and dorsomedial prefrontal cortices are activated during the performance of a variety of cognitive tasks that require working memory or goaldirected behavior (Fuster 1997). Hence, it is apparent that the effects of cocaine and associated cues extend beyond the limbic system to engage brain areas underlying complex cognitive processes. In the present study, the same neuroanatomical regions as reported in humans subjects were activated during cocaine self-administration and extinction in rhesus monkeys, establishing strong validity for the nonhuman primate model employed. Elevations in rates of glucose utilization in the same brain areas following cocaine self-administration in rhesus monkeys have also been reported (Porrino et al. 2002). Obviously, self-reports of drug craving cannot be obtained in animal studies. However, the distinct pattern of brain activation observed in the present study may provide a novel functional measure to assess interventions designed to attenuate cue-induced changes in brain activity.
In summary, this is the first study to use functional brain imaging to document acute cocaine-induced changes in brain activity during active drug use in nonhuman primates. There are significant challenges associated with the conduct of imaging in behaving nonhuman primates that impose limitations in the experimental design and interpretation of results. Subjects must undergo extensive periods of training and habituation to immobilization. The establishment of reliable stimulus control of behavior in a technically demanding environment is difficult. Moreover, the costs associated with PET imaging can be prohibitive. Accordingly, the current study was restricted to a single dose of cocaine in each experimental condition. The qualitative differences observed may have been related, in part, to differences in the effective concentration of cocaine. The interpretation of results clearly would benefit from parametric manipulation of drug dose. In addition, the use of PET imaging and O15 water imposes limitations on the temporal resolution of brain activation and the identification of underlying neural circuitry. While fMRI allows for continuous measurement of cerebral blood flow with outstanding temporal resolution, the technology and environment create additional challenges for awake animal imaging. Despite the inherent challenges and limitations, the results obtained in the present study are consistent with studies reporting the acute effects of cocaine or cocaine cues on brain activity in humans. The identification of neural circuits underlying the direct pharmacological and conditioned stimulus effects of cocaine may be highly relevant toward efforts to develop pharmacological treatments for cocaine addiction. There is recent evidence to suggest that the pattern and time course of brain activation in response to cocaine cues may predict treatment outcomes in human addicts (Kosten et al. 2006). Moreover, pretreatments with a selective serotonin transporter inhibitor were effective in blocking cocaine-induced brain activation in rhesus monkeys (Howell et al. 2002). It remains to be determined whether similar pharmacological treatments will be equally effective in attenuating cue-induced brain activation.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Tango Howard, James Jordan, Delicia Votaw, Kevin Murnane, and Peggy Plant. This research was supported by US Public Health Service Grants DA10344, DA00517, and RR00165 (Division of Research Resources, National Institutes of Health).
Contributor Information
Leonard L. Howell, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA; Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA, USA; Department of Pharmacology, Emory University, Atlanta, GA, USA
John R. Votaw, Department of Radiology, Emory University, Atlanta, GA, USA
Mark M. Goodman, Department of Radiology, Emory University, Atlanta, GA, USA
Kimberly P. Lindsey, McLean Hospital, Belmont, MA, USA
References
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex. Anatomy, physiology and neuropsychology of the frontal lobe. 3rd edn. Raven; New York: 1997. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cueinduced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Henry TR, Votaw JR, Pennell PB, Epstein CM, Bakay RA, Faber TL, Grafton ST, Hoffman JM. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999;52(6):1166–1173. doi: 10.1212/wnl.52.6.1166. [DOI] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. CRC; Boca Raton: 2001. pp. 91–110. [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods. 2001;106:161–169. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 2002;159:154–160. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Trivializing dependence. Br J Addict. 1990;85:1425–1427. doi: 10.1111/j.1360-0443.1990.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Katz JL, Goldberg SR. Second-order schedules of drug injection: implications for understanding reinforcing effects of abused drugs. Adv Subst Abuse. 1991;4:205–223. [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Lowe JV, Humphries D. Acute changes in cranial blood flow after cocaine hydrochloride. Biol Psychiatry. 1996;40:609–616. doi: 10.1016/0006-3223(95)00033-x. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W, Kutner MH. Applied linear statistical models: regression, analysis of variance, and experimental designs. 3rd edn. Irwin; Homewood: 1990. [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding Y-S, Pappas N. Association of methylphenidate-induced craving with changes in right striatoorbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding Y-S, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006a;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. NeuroImage. 2006b;32:1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw JR, Li HH. Analysis of PET neurofunctional mapping studies. J Cereb Blood Flow Metab. 1995;15(3):492–504. doi: 10.1038/jcbfm.1995.61. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Grafton ST, Hoffman JM. Calculation of the probability that an activation site has occurred by chance. In: Carson RE, Daube-Witherspoon ME, Herscovitch P, editors. Quantitative functional brain imaging with positron emission tomography. Academic; New York: 1998. pp. 229–235. [Google Scholar]
- Votaw JR, Faber TL, Popp CA, Henry TR, Trudeau JD, Woodard JL, Mao H, Hoffman JM, Song AW. A confrontational naming task produces congruent increases and decreases in PET and fMRI. Neuroimage. 1999;10(4):347–356. doi: 10.1006/nimg.1999.0471. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intra-subject, intramodality validation. J Comput Assist Tomogr. 1998a;22(1):139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998b;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]


