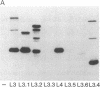
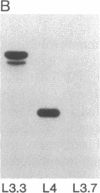
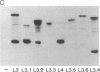
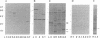Abstract
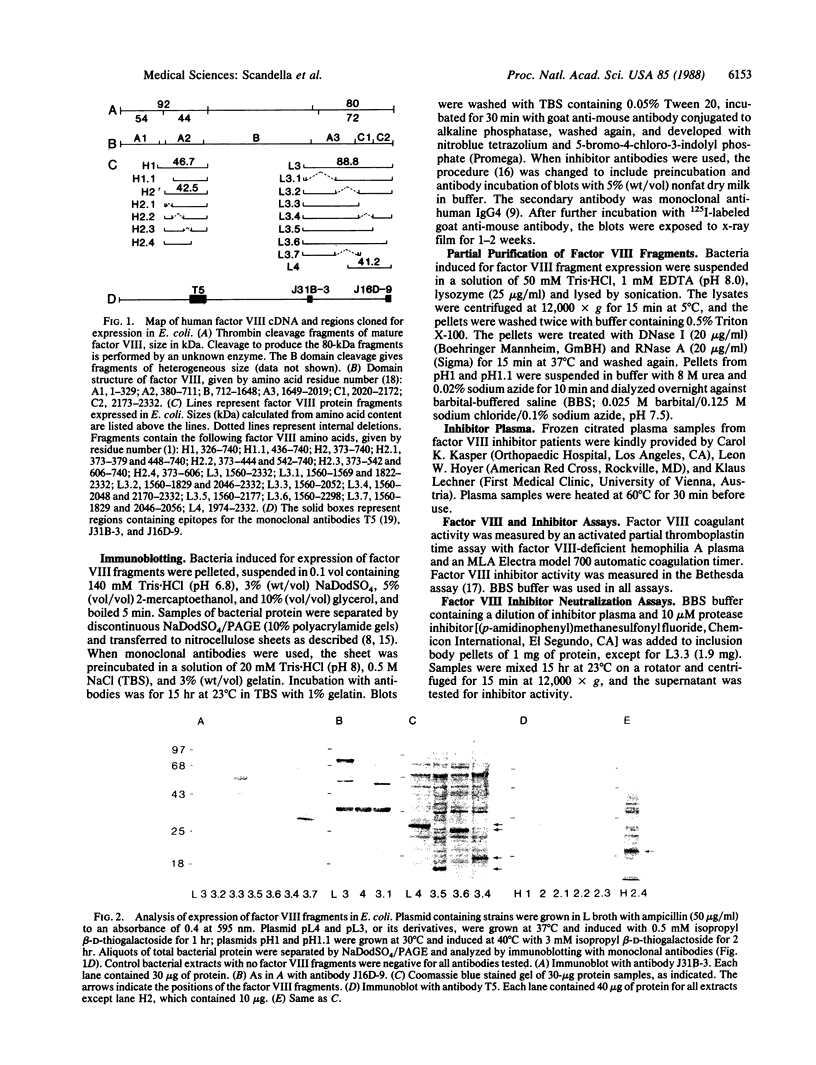
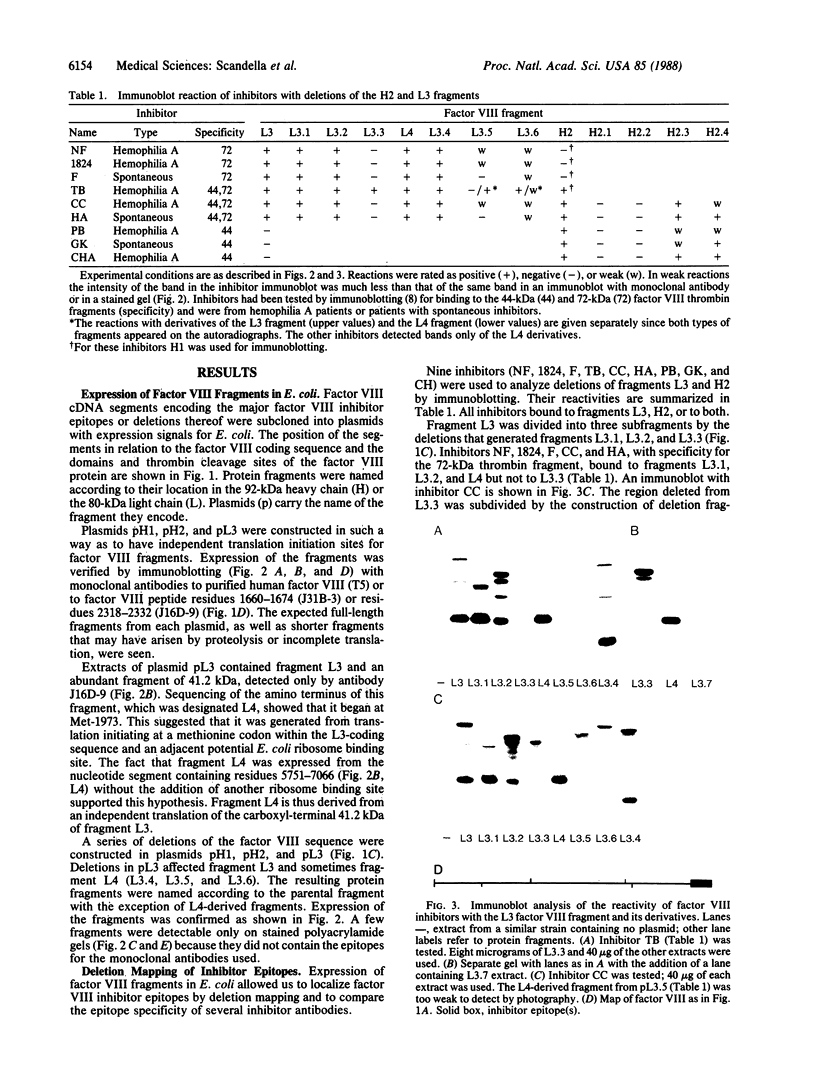
Epitopes for antibodies that inhibit factor VIII procoagulant protein were analyzed by deletion mapping of factor VIII protein fragments expressed in Escherichia coli. A human factor VIII cDNA clone was used to generate E. coli expression vectors encoding fragments containing the 80-kDa factor VIII light chain (A3, C1, and C2 domains) and the 44-kDa carboxyl-terminal half of the factor VIII heavy chain (A2 domain). A series of deletions of each fragment was constructed and tested by immunoblotting for the binding of alloantibody and autoantibody inhibitors. Analysis of derivatives of the 80-kDa fragment showed that six inhibitors recognized a major epitope(s) within the carboxyl-terminal 17.3 kDa of factor VIII. These inhibitors also recognized weaker epitopes nearby and one inhibitor recognized epitopes scattered throughout the 80-kDa fragment. Deletions within the heavy chain fragment revealed one epitope-containing region confined to the amino-terminal 18.3 kDa recognized by six inhibitors. Bacterially produced factor VIII fragments containing the major epitopes were capable of neutralizing inhibitors in vitro but fragments containing weaker or no epitopes did not. These data suggest a potential therapeutic use of factor VIII fragments for neutralization of inhibitor antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Andersson L. O., Forsman N., Huang K., Larsen K., Lundin A., Pavlu B., Sandberg H., Sewerin K., Smart J. Isolation and characterization of human factor VIII: molecular forms in commercial factor VIII concentrate, cryoprecipitate, and plasma. Proc Natl Acad Sci U S A. 1986 May;83(9):2979–2983. doi: 10.1073/pnas.83.9.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croissant M. P., Zuzel M., Allain J. P. Heterogeneity of autoantibodies to factor VIII: differences in specificity for apparently distinct antigenic determinants of factor VIII coagulant protein. Blood. 1983 Jul;62(1):133–140. [PubMed] [Google Scholar]
- Eaton D. L., Vehar G. A. Factor VIII structure and proteolytic processing. Prog Hemost Thromb. 1986;8:47–70. [PubMed] [Google Scholar]
- Eaton D., Rodriguez H., Vehar G. A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986 Jan 28;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Roberts J. R., Holland L. Z., Zimmerman T. S. Human factor VIII procoagulant protein. Monoclonal antibodies define precursor-product relationships and functional epitopes. J Clin Invest. 1985 Jul;76(1):117–124. doi: 10.1172/JCI111933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher C. A., Roberts J. R., Zimmerman T. S. Thrombin proteolysis of purified factor viii procoagulant protein: correlation of activation with generation of a specific polypeptide. Blood. 1983 Apr;61(4):807–811. [PubMed] [Google Scholar]
- Fulcher C. A., de Graaf Mahoney S., Roberts J. R., Kasper C. K., Zimmerman T. S. Localization of human factor FVIII inhibitor epitopes to two polypeptide fragments. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7728–7732. doi: 10.1073/pnas.82.22.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher C. A., de Graaf Mahoney S., Zimmerman T. S. FVIII inhibitor IgG subclass and FVIII polypeptide specificity determined by immunoblotting. Blood. 1987 May;69(5):1475–1480. [PubMed] [Google Scholar]
- Gawryl M. S., Hoyer L. W. Inactivation of factor VIII coagulant activity by two different types of human antibodies. Blood. 1982 Nov;60(5):1103–1109. [PubMed] [Google Scholar]
- Letter: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975 Dec 15;34(3):869–872. [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Rotblat F., Tuddenham E. G. Immunologic studies of factor VIII coagulant activity (VIII:C) 1. Assays based on a haemophilic and an acquired antibody to VIII:C. 1981 Feb 15-Mar 1Thromb Res. 21(4-5):431–445. doi: 10.1016/0049-3848(81)90144-4. [DOI] [PubMed] [Google Scholar]
- Sarver N., Ricca G. A., Link J., Nathan M. H., Newman J., Drohan W. N. Stable expression of recombinant factor VIII molecules using a bovine papillomavirus vector. DNA. 1987 Dec;6(6):553–564. doi: 10.1089/dna.1987.6.553. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Capon D. J., Simonsen C. C., Eaton D. L., Gitschier J., Keyt B., Seeburg P. H., Smith D. H., Hollingshead P., Wion K. L. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984 Nov 22;312(5992):330–337. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]