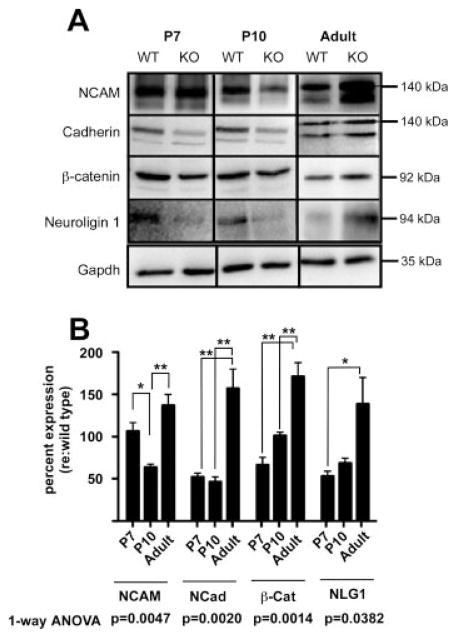
Figure 3.
α9 nAChR regulates the expression of trans-synaptic adhesion proteins. (A) Total cochlear lysates from α9 null and wild-type control mice at P7, P10, and adult (2 months) ages were probed by Western blotting with antibodies against trans-synaptic and signaling proteins as indicated. In the case of the cadherins and the NCAM immunoblots, the antibodies detected previously recognized isoforms. Note that only the 140 kDa and 120 kDa form of NCAM in the cochlea, not the 180 kDa isoform, were detected. Similarly, the pan Cadherin antibody recognized multiple bands in the immunoblot. Based on mass, the upper band was identified as N-Cadherin, while the lower band was identified as E-Cadherin. Densitometric values were obtained only for the 140 kDa isoform of NCAM because of its expression over all the ages examined, and for N-Cadherin due to its role in synapse formation/maintenance. All data were normalized to densitometric values of loading control GAPDH. (B) The dynamic change in expression of the adhesion and adhesion-related proteins expressed by α9 null mice is displayed as percent change relative to wild-type levels in the time series bar graph. Quantification of the change in protein expression levels in α9 null relative to GAPDH was then normalized to the wild type expression GAPDH (and multiplied by 100) to better visualize changes in dynamics of protein expression. A value of 100 would therefore represent no change from the wild type expression values. One-way ANOVA and Tukey post-hoc multiple comparisons tests were used to assess changes in protein expression of α9 nulls normalized to wild type levels over time (*p < 0.05, **p < 0.005).