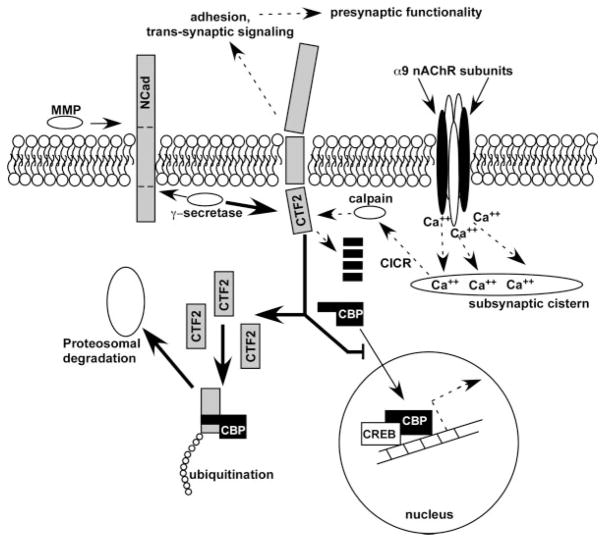
Figure 8.
Summary of potential mechanism by which altered OC nAChR activity may drive structural and functional changes observed in cochleae of α9 null mice. N-Cadherin is cleaved first by ADAM10, a metaloprotease enzyme, to generate an N-term fragment of N-Cadherin. Following MMP cleavage, the γ-secretase/PS1 complex cleaves the C-terminal fragment (CTF) to release the CTF2 fragment into the cytoplasm. CTF2 has been shown to be proteolytically cleaved by calcium-activated calpain, but if intact can also bind CREB-binding protein, where the entire complex is then ubiquitinated and sent for proteosomal degradation. Loss of CBP via this pathway would alter CREB-associated transcriptional activity. In α9 nAChR subunit null mice, less calcium enters the hair cells as a result of loss of ACh-inducible activity (dashed lines below nAChR complex). This would result in a loss of calcium-induced calcium release (CICR) from the subsynaptic cistern, and a decrease in calpain activity. The result would be an accumulation of CTF2 upon the normal γ-secretase activity acting on the over expressed levels of N-Cadherin (thick solid arrow). Accumulated CTF2 in the null mice binds a greater proportion of CBP, sending it off for degradation and at the same time inhibiting translocation of normal levels of CBP into the nucleus. Hyperactivity of the α9KI channel may induce greater calpain activity as a result of excessive calcium signaling, resulting in a greater proportion of CTF2 degradation in adults before it binds to CBP. Thus, CBP levels may be higher in adults, reflecting the early stage innervation of the α9 nulls in which exuberant contacts were observed between the OC fibers and the OHCs, and potentially explaining the hyper-innervation demonstrated in Figure 7. Dashed lines with arrows represents downregulated pathways; thin lines with arrows represents normally active pathways; thick lines with arrows represents upregulated overactive pathways; black elements represents downregulated or missing proteins (e.g. α9 nAChR subunit, CBP); gray elements represents upregulated proteins.