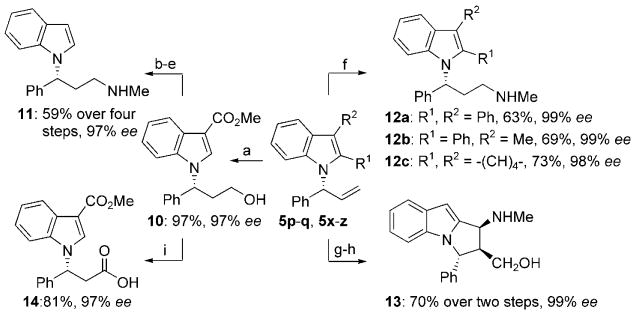
Scheme 2.
Syntheses of enantioenriched 3-(1H-indol-1-yl)-N-methyl-3-arylpropan-1-amines 11 and 12a-c, dihydropyrrolo[1,2-a]indole 13, and indol-1-yl propanonic acid 14 from N-allylindoles. N-Allylindoles: 5p, R1=CHO, R2=H, 99% ee; 5q, R1=H, R2=CO2Me, 97% ee; 5x, R1, R2=Ph, 99% ee; 5y, R1=Ph, R2=Me, 99% ee; 5z, R1, R2=-(CH)4-, 98% ee. Reaction conditions: a) 5q, 9-BBN, THF, −78°C to RT, then H2O2, 3m NaOH, EtOH, 0°C to RT, 97%, 97% ee; b) KOH, MeOH, reflux; c) PhBr, reflux, 68% over two steps; d) PPh3, CCl4, 0°C→RT, 91%; e) MeNH2, EtOH, 90°C, 95%, 97% ee; f) 5x–z, [Cp2Zr(H)Cl], THF, RT, then CH3NHOSO3H, 60°C (see Scheme 2 for results); g) 5p, MeNHOH·HCl, NaOAc, THF, reflux, 83%, 9:1 regioselectivity, >99:1 d.r.; h) Zn, AcOH, H2O, 60°C, 87%, 99% ee; i) PhI(OAc)2, TEMPO, NaHCO3, MeCN/H2O (1:1), 81%, 97% ee. 9-BBN=9-borabicyclo[3.3.1]nonane, TEMPO=2,2,6,6-tetramethyl-1-piperidinyloxy free radical.