Table 1.
Iridium-catalyzed enantioselective allylation of ethyl indole-2-carboxylate 4a with allylic carbonates 3a–k.[a]
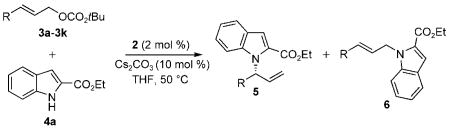 | |||||
|---|---|---|---|---|---|
| Entry | 3 (R) | 5 | 5/6[b] | Yield [%][c] | ee [%][d] |
| 1 | 3a (Ph) | 5a | 97:3 | 89 | 99 |
| 2 | 3b (4-MeOC6H4) | 5b | 99:1 | 88 | 99 |
| 3 | 3c (4-BrC6H4) | 5c | 97:3 | 87 | 99 |
| 4[e] | 3d (4-F3CC6H4) | 5d | 91:9 | 85 | 99 |
| 5 | 3e (3-MeOC6H4) | 5e | 98:2 | 95 | 98 |
| 6[f] | 3 f (2-MeOC6H4) | 5 f | 95:5 | 72 | 96 |
| 7[f] | 3g (2-furyl) | 5g | 91:9 | 85 | 99 |
| 8[f,g] | 3h ((E)-CH=CHCH3) | 5h | 99:1 | 86 | 99 |
| 9[f] | 3i (n-propyl) | 5i | 94:6 | 92 | 99 |
| 10[f] | 3j (CH2OBn) | 5j | 77:23 | 70 | 98 |
| 11[e] | 3k (cyclohexyl) | 5k | 87:13 | 54 | 99 |
See the Supporting Information for experimental details.
Determined by 1H NMR analysis of the crude reaction mixture.
Yield of isolated 5.
Determined by chiral HPLC methods.
Used 4 mol% 2 as the catalyst.
Reaction was run at room temperature.
Enantiomeric excess was determined after reduction with LiAlH4.
