Table 2.
Iridium-catalyzed N-allylation of 2-substituted, 3-substituted, and 2,3-disubstituted indoles 4b–p with tert-butyl cinnamyl carbonate 3a.[a]
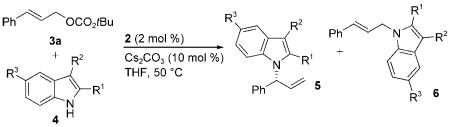 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | 4 | R1 | R2 | R3 | 5 | 5/6[b] | Yield [%][c] | ee [%][d] |
| 1 | 4b | CO2Et | H | OMe | 5l | 97:3 | 88 | 99 |
| 2 | 4c | CO2Et | H | F | 5m | 94:6 | 84 | 99 |
| 3 | 4d | CO2Et | H | Cl | 5n | 95:5 | 91 | 99 |
| 4 | 4e | CO2Et | H | NO2 | 5o | 94:6 | 90 | 99 |
| 5 | 4f | CHO | H | H | 5p | 98:2 | 89 | 99 |
| 6 | 4g | H | CO2Me | H | 5q | 96:4 | 84 | 97 |
| 7 | 4h | H | CHO | H | 5r | 94:6 | 87 | 96 |
| 8 | 4i | H | C(O)Me | H | 5 s | 98:2 | 88 | 96 |
| 9 | 4j | H | CN | H | 5t | 96:4 | 93 | 96 |
| 10 | 4k | H | Ph | H | 5u | 99:1 | 21 | 97 |
| 11 | 4 l | CHO | Me | H | 5v | 98:2 | 82 | 99 |
| 12 | 4m | Ph | CHO | H | 5w | 96:4 | 93 | 97 |
| 13 | 4n | Ph | Ph | H | 5x | 98:2 | 95 | 99 |
| 14 | 4o | Ph | Me | H | 5y | 98:2 | 89 | 99 |
| 15 | 4p | -(CH)4- | H | 5z | 98:2 | 88 | 98 | |
See the Supporting Information for experimental details.
Determined by 1H NMR analysis of the crude reaction mixture.
Yield of isolated 5.
Determined by chiral HPLC methods.
Methyl cinnamyl carbonate was used as the electrophile.
