Table 2.
Scope of the allylic etherification with various alcohols.[a]
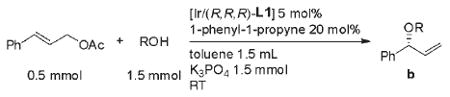 | ||||
|---|---|---|---|---|
| Entry | R | t [h] | b:l[b] | Yield [%], ee[c] or de |
| 1 | Bn | 22 | 99:1 | 68, 93% ee |
| 2 | n-hexyl | 40 | 99:1 | 71, 91% ee |
| 3[d] | n-hexyl | 20 | 84:16 | 77, 94% ee |
| 4[d] | cyclo-hexyl | 40 | 99:1 | 68, 93% ee |
| 5 | N-Boc-4-piperidinyl[e] | 50 | 99:1 | 66, 90% ee |
| 6 | (S)-1-phenylethanol | 80 | 99:1 | 67, 88% de[f] |
| 7[g] | (S)-1-phenylethanol | 40 | 99:1 | 63, 90% de |
| 8 | TBDMS[h] | 40 | 98:2 | 85, 98% ee |
All times, ratios of b:l, isolated yields and ee or de values are averages from two independent runs.
Ratio of b:l determined by 1H NMR analysis of the crude reaction mixture.
Determined by HPLC.
Alcohols (2.5 mmol) and K3PO4 (2.5 mmol) were used.
Boc=tert-butoxycarbonyl.
d.r. 94:6.
(S,S,S)-L1 was used; d.r.=95:5.
TBDMS=tert-butyldimethylsilyl.
