Table 1.
Initial catalyst screening for molybdenum-catalyzed ARCM reactions of 6.a
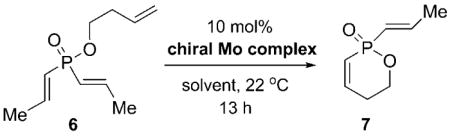 | ||||
|---|---|---|---|---|
| Entry | Chiral Mo Complex | Solvent | Conv. [%]b | ee [%]c |
| 1 | 1 a | C6H6 | <2 | – |
| 2 | 1 a | CH2Cl2 | <2 | – |
| 3 | 1 b | C6H6 | 45 | 51 |
| 4 | 1 b | CH2Cl2 | 63 | 60 |
| 5 | 2 | C6H6 | 15 | −22 |
| 6 | 2 | CH2Cl2 | 49 | 11 |
| 7 | 3 a | C6H6 | 38 | 60 |
| 8 | 3 a | CH2Cl2 | 32 | 53 |
| 9 | 3 b | C6H6 | <2 | – |
| 10 | 3 b | CH2Cl2 | <2 | – |
| 11 | 4 a | C6H6 | 51 | 16 |
| 12 | 4 a | CH2Cl2 | 65 | 11 |
All reactions performed under a nitrogen atmosphere.
Conversion into the desired product was measured by 1H NMR analysis (400 MHz) of the unpurified reaction mixture; traces of homodimer derived from reaction of terminal olefins were present.
Determined by GLC analysis of the purified material; see the Supporting Information for details.
