Table 3.
Catalytic Enantioselective RCM of Triene 2 Promoted by Stereogenic-at-Mo Complexesa
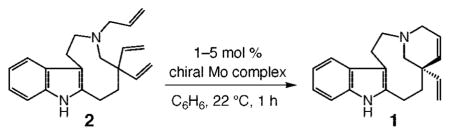 | ||||
|---|---|---|---|---|
| entry | chiral complex; mol % | conv (%);b yield (%)c | erd | ee (%)d |
| 1 | 36a; 1 | <10; nd | nd | nd |
| 2 | 36b; 1 | 24; nd | nd | nd |
| 3 | 36b; 5 | >98; 60 | 83.5:16.5 | 67 |
| 4 | 37a; 1 | <5; nd | – | – |
| 5 | 37b; 1 | >98; 83 | 97.5:2.5 | 95 |
| 6 | 38; 1 | 19; nd | nd | nd |
Reactions were performed under N2 atmosphere; see the Supporting Information for experimental details. nd = not determined.
Conversions (based on the amounts of unreacted substrate and product formed) were determined through analysis of 400 MHz 1H NMR spectra of the unpurified mixtures.
Yield of 1 after purification.
Enantiomer ratios (er) and enantioselectivities (ee) were determined by HPLC analysis; see the Supporting Information for details.
