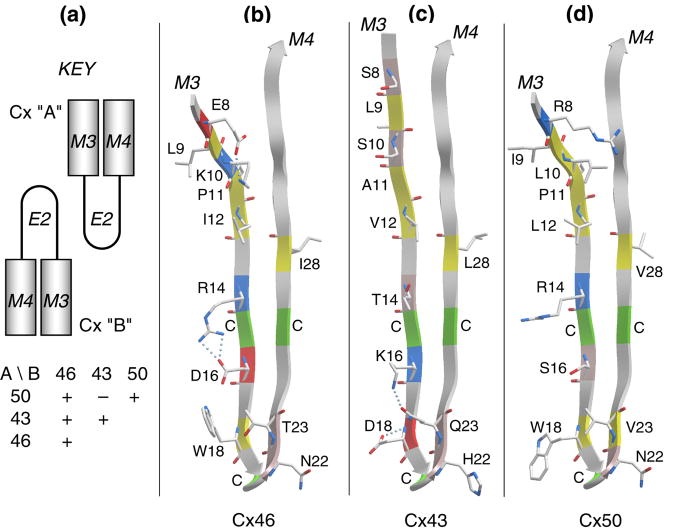
Figure 4.
The E2 loop is a primary determinant of isoform compatibility. (a) A schematic key to show the compatibilities in pairing of heterotypic channels formed by C×46, C×43 and C×50. (b), (c) and (d) show a comparison of the E2 loops of C×46, C×43 and C×50, respectively. Only amino acids that differ in at least one of the three isoforms are shown in a stick representation. Cysteines are indicated with C and colored green on the ribbon. Residue coloring is: yellow, hydrophobic; pink, polar; red, acidic; blue, basic. Note that the C-terminal halves of the loops are highly conserved, suggesting that selectivity is dictated by the N-terminal regions of the loops. As discussed in the text, the hydrogen-bonding pattern near the β hairpin of C×43, along with its H22, may account for its incompatibility with C×50. This analysis was based on the 3 localized regions that are predicted to be β strands and did not depend on the complete β-turn-β fold depicted in the figure.