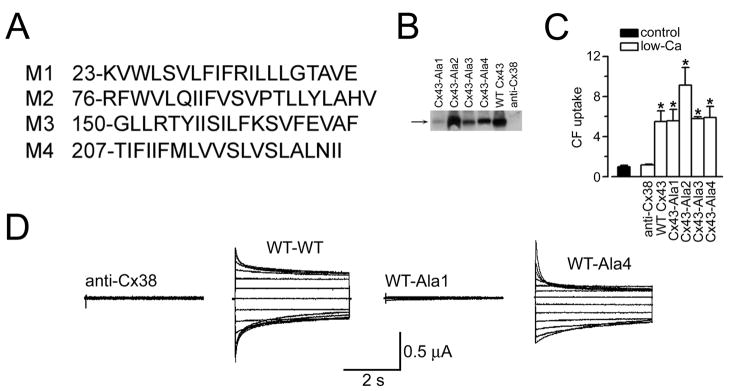
Figure 6.
Formation of regulated gap junction channels and hemichannels by C×43 poly-Ala mutants (adapted from Bao et al., 2005). (A) Sequences of wild-type C×43 TM helices replaced with poly-Ala sequences. The position of the first residue of each sequence is indicated. (B) Immunoblots of plasma membranes from frog oocytes injected with anti-C×38 antisense oligonucleotide alone (anti-C×38) or in combination with cRNA coding for WT rat C×43 (WT C×43) or the C×43 poly-Ala mutants (C×43-Ala1 to C×43-Ala4). Data representative of 3 similar blots. (C) Uptake of CF via hemichannels. Data were normalized to the uptake in low-[Ca2+] solution in oocytes expressing WT C×43. Data are means ±SEM of 30–40 oocytes per condition. *P<0.05 compared to the control value, in the presence of 1.8 mM Ca2+. Units in the vertical axis are relative fluorescence values. (D) Formation of gap junction channels. Typical gap junction currents. Oocytes injected with anti-C×38 antisense oligonucleotide (anti-C×38), or expressing WT C×43 or poly-Ala mutants, were paired, and the junctional currents (Ij) were measured upon 4-s transjunctional voltage (Vj) steps between −120 and 120 mV, applied in 20-mV increments from a holding voltage of −60 mV. Four sets of records are shown, which Ij correspond to anti-C×38-anti-C×38, WT-WT, WT-Ala1 and WT-Ala4 oocyte pairs. The recordings are representative of 6–10 experiments per condition, obtained using the dual-electrode voltage-clamp technique (Bao et al., 2004b).