Summary
In the last decade, the fruit fly Drosophila melanogaster, highly accessible to genetic, behavioral and molecular analyses, has been introduced as a novel model organism to help decipher the complex genetic, neurochemical, and neuroanatomical underpinnings of behaviors induced by drugs of abuse. Here we review these data, focusing specifically on cocaine-related behaviors. Several of cocaine's most characteristic properties have been recapitulated in Drosophila. First, cocaine induces motor behaviors in flies that are remarkably similar to those observed in mammals. Second, repeated cocaine administration induces behavioral sensitization a form of behavioral plasticity believed to underlie certain aspects of addiction. Third, a key role for dopaminergic systems in mediating cocaine's effects has been demonstrated through both pharmacological and genetic methods. Finally, and most importantly, unbiased genetic screens, feasible because of the simplicity and scale with which flies can be manipulated in the laboratory, have identified several novel genes and pathways whose role in cocaine behaviors had not been anticipated. Many of these genes and pathways have been validated in mammalian models of drug addiction. We focus in this review on the role of LIM-only proteins in cocaine-induced behaviors.
Keywords: Drosophila, cocaine sensitivity, dopamine, LMO, Lmo4 gene-trap
Introduction
Cocaine, a naturally occurring plant alkaloid, is the prototype addictive psychomotor stimulant. It elicits a variety of acute behavioral changes ranging from mood elevation, disinhibition, and motor activation at low doses to compulsive stereotypies and psychosis at higher doses (Gawin, 1991). Long–term cocaine use generally results in tolerance to many of its subjective effects, an increased craving towards the drug, and eventually to drug abuse and addiction. Cocaine's primary mechanism of action is to bind and inhibit plasma membrane monoamine transporters, thereby increasing synaptic monoamine neurotransmitter levels and potentiating their actions. In mammalian animal models, the acute response to cocaine is predominantly observed as enhanced locomotor activity and stereotypic behaviors. This locomotor-stimulant effect of cocaine is mediated primarily by an inhibition of the dopamine transporter (DAT), as mice lacking DAT show enhanced levels of baseline activity that are insensitive to cocaine administration (Giros et al., 1996). Regulation of the rewarding effects of cocaine are, however, more complex, involving, in addition to DAT, the serotonin transporter (SERT) (Sora et al., 1998; Sora et al., 2001) as well as many additional genes. How the acute stimulant effects of cocaine relate to the long-term changes that underlie addiction is poorly understood. However, emerging evidence suggests that the mechanisms that regulate the acute stimulant effects of psychostimulants are also involved in determining their rewarding properties (Laakso et al., 2002). For example, the locomotor activity of mice lacking both DAT and SERT is unaltered by cocaine administration; these mice also fail to develop conditioned preference for cocaine, an assay that measures the rewarding effects of the drug (Sora et al., 2001). Conversely, mice lacking FosB or overexpressing ΔfosB, which are highly sensitive to the psychomotor stimulant effects of cocaine, also show enhanced place preference for cocaine (Hiroi et al., 1997; Kelz et al., 1999). It is therefore likely that a mechanistic understanding of the relatively simple process of acute drug-induced locomotor stimulation may provide valuable clues about the molecular mechanisms underlying drug reward, reinforcement, and addiction.
Drosophila as a model
Drosophila, one of the most intensively studied organisms in biology, has provided crucial insights into developmental and cellular processes that are conserved with mammals, including humans. Flies have a relatively sophisticated nervous system (approximately 300,000 neurons) and are capable of many complex behaviors (Hall, 1994; DeZazzo and Tully, 1995; Hall, 1998; Sokolowski, 2001). They are easy and inexpensive to rear in the laboratory and their life cycle is only approximately two weeks. The major advantage of flies is the simplicity and scale with which they can be manipulated genetically. A century of fundamental genetic analysis has led to the generation of a large number of sophisticated genetic tools (Lindsley, 1992; Rubin and Lewis, 2000), and the last few decades have witnessed the development of many powerful molecular genetic techniques that allow germline transformation (Rubin and Spradling, 1982; Spradling and Rubin, 1982), the use of transposable elements as mutagens (Engels, 1983), homologous recombination (Rong and Golic, 2000), and double stranded RNA-mediated gene expression interference (RNAi) (Carthew, 2001; Kalidas and Smith, 2002). Moreover, an analysis of the Drosophila euchromatin sequence revealed a high degree of molecular similarity between flies and mammals (Adams et al., 2000; Myers et al., 2000; Rubin et al., 2000). For example, Drosophila has most – if not all – major neurotransmitters, molecules involved in synaptic vesicle release and recycling, receptors and channels for neurotransmission, and signal transduction mechanisms involved in neural function in mammals (Littleton and Ganetzky, 2000; Lloyd et al., 2000). However, there are several notable differences. For example, flies use acetylcholine instead of glutamate as the major excitatory CNS neurotransmitter, and glutamate instead of acetylchole at the neuromuscular junction. In addition, flies lack noradrenaline with octopamine fulfilling its many roles (Roeder, 1999). Importantly, genes implicated directly or indirectly in the actions of abused drugs are, for the most part, conserved. In addition to the above-mentioned genetic tools, multiple techniques exist to alter the function of specific populations of nervous system cells, thus allowing the definition of the neuroanatomical loci that regulate behaviors of interest (Brand et al., 1994; Kitamoto, 2001; Osterwalder et al., 2001; Roman et al., 2001; Stebbins et al., 2001; Stebbins and Yin, 2001; McGuire et al., 2003; McGuire et al., 2004). In recent years, these powerful tools have been applied to the study of behaviors induced by drugs of abuse, including alcohol, cocaine, and nicotine, in Drosophila (Rothenfluh and Heberlein, 2002; Guarnieri and Heberlein, 2003; Wolf and Heberlein, 2003). As a consequence, the molecular genetic, neuroanatomical and neurochemical bases for responses to abused drugs in Drosophila are beginning to be understood, revealing a large degree of mechanistic conservation with mammalian systems.
Role of biogenic amine systems in Drosophila cocaine-induced behaviors
Flies synthesize the biogenic amines dopamine, octopamine and tyramine, as well as serotonin and possibly additional trace amines (Monastirioti, 1999). They also contain proteins involved in the pre-synaptic machinery needed for their synthesis, release and reuptake, as well as receptors and signaling pathways that mediate their pre- and post-synaptic effects (Littleton and Ganetzky, 2000; Lloyd et al., 2000). Interference with dopamine synthesis with the tyrosine hydroxylase (TH) inhibitor 3-iodo-tyrosine leads to reduced effectiveness of cocaine (Bainton et al., 2000). In addition, the application of cocaine solution directly to the ventral nerve cord of decapitated flies induces grooming and aberrant locomotion, an effect that is blocked by preadministration of a dopamine D1 receptor antagonist (Torres and Horowitz, 1998). Similar behavioral effects are seen after direct application of monoamines or dopamine receptor agonists to the ventral nerve cord in decapitated flies (Yellman et al., 1998). The finding that inhibition of synaptic transmission in dopaminergic and serotonergic neurons leads to cocaine hypersensitivity is therefore somewhat surprising (Li et al., 2000). However, because these neurons are silenced throughout development, compensatory adaptations, such as hypersensitivity of the postsynaptic receptors, may be responsible for the increased cocaine sensitivity. The latter is supported by the finding that direct application of the dopamine receptor agonist quinpirole to the ventral nerve cord of these flies induces an enhanced locomotor response (Li et al., 2000).
Flies (and insects in general) synthesize tyramine from tyrosine by the action of tyramine decarboxylase (Tdc); tyramine is converted into octopamine by tyramine-β-hydroxylase (Tβh) (Monastirioti, 1999). Flies that lack both neural tyramine and octopamine because of mutation in one of the tyramine decarboxylase-encoding genes, Tdc2, have dramatically reduced basal locomotor activity levels and are hypersensitive to an initial dose of cocaine (Hardie et al., 2007). In contrast, flies that contain no measurable neural octopamine and an excess of tyramine due to a null mutation in the tyramine-β-hydroxylase gene exhibit normal locomotor activity and cocaine responses (Hardie et al., 2007). Finally, a Drosophila isoform of the vesicular monoamine transporter VMAT (DVMAT-A) is expressed in both dopaminergic and serotonergic neurons in the adult Drosophila brain. Overexpression of DVMAT-A in these cells potentiates spontaneous stereotypic grooming and locomotion, effects that can be reversed by blocking DVMAT activity, and administration of a dopamine receptor antagonist. In addition, DVMAT-A overexpression decreases the fly's sensitivity to cocaine, suggesting that the synaptic machinery responsible for this behavior may be downregulated by DVMAT-A overexpression (Chang et al., 2006). Taken together, these results indicate that dopaminergic (and possibly serotonergic) systems and the trace-amine tyramine mediate acute cocaine-induced behaviors. Tyramine has also been implicated in cocaine sensitization (McClung and Hirsh, 1999), although more recent data from the Hirsh laboratory indicate that this connection is tenuous (Hardie et al., 2007). The recent cloning of mammalian G-coupled receptors that respond specifically to trace amines such as tyramine (Borowsky et al., 2001; Bunzow et al., 2001; Miller et al., 2005), suggests that tyramine may act as a neurotransmitter/modulator in mammals.
Interestingly, one of these mammalian receptor can be directly activated by amphetamine (Bunzow et al., 2001). These trace amine receptors may also alter cocaine-induced behaviors indirectly through modification of monoamine transporter function (Miller et al., 2005) or through inhibition of dopaminergic neuron function (Geracitano et al., 2004). It will be interesting to determine if these receptors are involved in cocaine-related behaviors in mammals.
Novel genes involved in cocaine induced behaviors in Drosophila
The data summarized above show that several of cocaine's most characteristic properties have been recapitulated in flies. First, cocaine induces motor behaviors in flies that are remarkably similar to those observed in mammals (McClung and Hirsh, 1998; Bainton et al., 2000). Second, repeated cocaine administration induces behavioral sensitization (McClung and Hirsh, 1998; Dimitrijevic et al., 2004) a form of behavioral plasticity believed to underlie certain aspects of addiction (Robinson and Berridge, 1993; Schenk and Partridge, 1997). Finally, a key role for dopaminergic systems in mediating cocaine's effects has been demonstrated through both pharmacological and genetic methods (Bainton et al., 2000; Li et al., 2000; Chang et al., 2006). More importantly, Drosophila studies have identified genes and pathways whose role in cocaine responsiveness had not been anticipated. For instance, flies with mutations in the circadian genes period (per), clock, cycle and doubletime reduce or eliminate behavioral sensitization to cocaine (Andretic et al., 1999). Subsequently, mice carrying mutations in one of the mouse per genes, mper1, were found to have defective cocaine sensitization and conditioned place preference (Abarca et al., 2002), highlighting a high degree of conservation in specific gene-behavior relationships between Drosophila and mammals.
In addition, several novel genes regulating cocaine-induced behavior have been identified by unbiased genetic screens for fly mutants with abnormal cocaine responses using the “crackometer”. The crackometer is a foot-long narrow cylinder into which naive or drug-treated flies are introduced; naive flies climb quickly to the top of the column due to their innate propensity for negative geotaxis and positive phototaxis, while cocaine-treated flies remain near the bottom of the cylinder. A “drug effect score” (DES) is calculated based on the fraction of flies that fail to climb, with a high DES reflecting a strong effect of the drug on climbing behavior (Bainton et al., 2000). The G-protein-coupled receptors encoded by the moody gene function in a subtype of glia to regulate the permeability of the blood-brain barrier (BBB) (Bainton et al., 2005; Schwabe et al., 2005). The enhanced sensitivity to cocaine observed in moody mutants does not appear to be due to altered cocaine accessibility to the nervous system, but, rather, to some homeostatic effect that a slightly leaky BBB has on nervous system physiology (Bainton et al., 2005). Consistent with Moody functioning in G-protein signaling, the fly homolog of RGS4, loco, encoding a regulator/inhibitor of G-protein signaling (Granderath et al., 1999), was identified as a mutant with decreased cocaine sensitivity that also shows a defective BBB (Bainton et al., 2005; Schwabe et al., 2005). Interestingly, the levels of RGS 4 are downregulated in the prefrontal cortex and striatum of rodents after acute and chronic administration of psychostimulants or morphine (Bishop et al., 2002; Gold et al., 2003; Schwendt et al., 2006; Schwendt et al., 2007). However, mice lacking RGS4 show normal responses to opiods (Grillet et al., 2005); further studies with these mice may uncover a role for RGS4 in the behavioral effects of psychostimulants. Finally, in mammals, claudin-5, a cell-adhesion molecule found in tight junctions of epithelial cells that form the BBB, has been implicated in normal BBB function (Nitta et al., 2003). Moreover, the human claudin-5 and RGS4 loci have been associated with vulnerability to schizophrenia (Chowdari et al., 2002; Sun et al., 2004). These observations warrant a closer examination of the role of the BBB, and the molecules that regulate its permeability, in nervous system function and the etiology of mental illness, including drug addiction.
white rabbit, encoding a Rho-family GTPase activating protein (Rho-GAP) was also identified by an unbiased genetic screen for mutants with reduced cocaine-, nicotine- and ethanol-sensitivity (Rothenfluh et al., 2006). Small GTPases of the Rho family act as molecular switches transducing extracellular signals to changes in the actin cytoskeleton (Etienne-Manneville and Hall, 2002; Meyer and Feldman, 2002). Their ability – and that of the molecules, such as RhoGAPs, that affect their activity – to dynamically regulate the actin cytoskeleton and, consequently, the reorganization of axonal and dendritic branches (Bonhoeffer and Yuste, 2002; Luo, 2002), makes them ideally suited to regulate synaptic plasticity and behavior. Indeed, defects in these processes has been linked with certain forms of mental retardation, conditions commonly associated with abnormalities in dendrite and dendritic spine morphology. Moreover, several genes implicated in non-syndromic mental retardation are directly linked to Rho-type GTPase signaling (Ramakers, 2002; Ropers and Hamel, 2005). Importantly, cocaine has been shown to affect proper actin dynamics, which in turn has been postulated to modulate cocaine-induced reinstatement of drug seeking (Toda et al., 2006).
Role of the Drosophila LIM-only gene, dLmo, in cocaine sensitivity
A genetic screen for Drosophila mutants with altered acute responses to cocaine identified mutations in the Drosophila LIM-only gene, dLmo, isolated due to their increased sensitivity to cocaine–induced loss of negative geotaxis in the “crackometer” (Tsai et al., 2004). The LIM motif, a cystein–rich zinc-coordinating domain that mediates protein-protein interactions, was originally discovered as a component of LIM-homeodomain (LIM-HD) transcription factors (Dawid et al., 1998). LMO proteins, are nuclear proteins composed almost entirely by two tandem LIM domains. The dLMO protein modulates the function of the LIM-HD factor encoded by the apterous gene by competing with the binding of Apterous with its cofactor Chip (Fig. 2) (Milan et al., 1998). Flies carrying loss-of-function mutations in dLmo display increased sensitivity to the effects of cocaine, while gain-of-function mutations (in which dLmo is overexpressed), show the converse effect, a reduced response to the drug (Tsai et al., 2004). This inverse relationship between dLmo gene activity and drug responsiveness suggests that dLmo regulates the expression of genes that might play a direct role in controlling cocaine responses.
Figure 2. Mechanism of dLmo function.
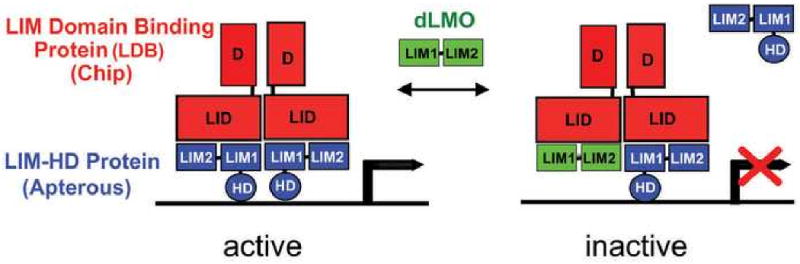
In Drosophila one LMO protein is encoded by the dLmo locus, and has been studied primarily for its role in the development of the fly wing, which has led to a model for its function (Milan et al., 1998; Shoresh et al., 1998; Zeng et al., 1998). dLMO protein inhibits the activity of the LIM–HD transcription factor Apterous through its interactions with the co–activator Chip (the fly homologue of Clim/Ldb1/NL1) (Fernandez-Funez et al., 1998; Milan and Cohen, 1999; van Meyel et al., 1999; Weihe et al., 2001). Binding of Chip to Apterous leads to its dimerization and activation. dLMO competes for Chip binding; consequently, increased dLMO levels lead to inhibition of Apterous/LIM-HD function, while reduced dLMO levels cause increased Apterous/LIM-HD activity.
dLmo expression is prominent in several distinct brain regions, including the antennal lobes and the the mushroom bodies, which are major brain centers involved in olfaction and olfactory conditioning, respectively (Stocker, 1994; Zars, 2000). In addition, dLmo is expressed prominently in the ventral lateral neurons (LNvs) (Fig. 3), the major pacemaker cells regulating circadian locomotor rhythmicity in flies (Renn et al., 1999). Targeted expression of dLmo in these different brain regions revealed that the altered cocaine responses are due to differential dLmo expression in the LNvs (Tsai et al., 2004). LNvs express the neuropeptide pigment-dispersing factor (PDF), the primary functional output of the LNvs in the regulation of circadian rhythms (Renn et al., 1999). Consistent with a dysfunction of these pacemaker neurons in dLmo mutants, the mutant flies also show somewhat altered circadian locomotor rhythms (Tsai et al., 2004), adding to mounting evidence in Drosophila and mice supporting a role for circadian genes in cocaine-related behaviors. The finding that cocaine actions are modulated by neurons critical for normal circadian locomotor rhythmicity suggests a basis for this overlap.
Figure 3. Expression of dLmo in PDF-positive circadian pacemaker cells (LNvs) in Drosophila.
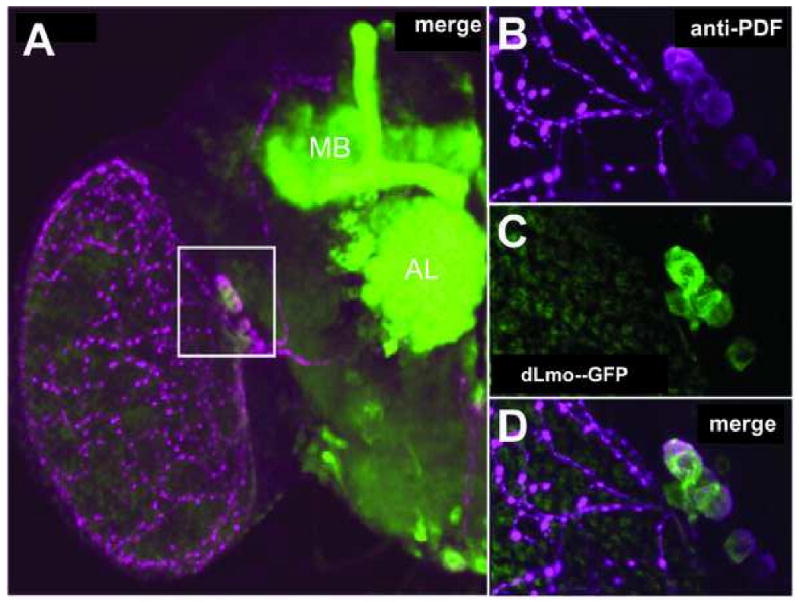
(A) Confocal image of an adult brain hemisphere (midline to the right), in which GFP expression is under the control of dLmo regulatory sequences (Tsai et al., 2004), double-stained with antibodies that recognizes PDF (magenta) and GFP (green). Overlap between GFP and PDF expression is observed in the LNvs (white box). GFP expression is also seen in the mushroom body (MB) and antennal lobe (AL). (B-D) Higher magnification views of the LNvs (white box in panel A). (Tsai et al., 2004; reprinted with permission)
The observation that dLmo functions in the PDF-expressing LNvs to regulate cocaine sensitivity, together with the finding that dLmo mutants show abnormal circadian locomotor rhythms, suggested that the pathways regulating cocaine sensitivity may be under the control of the circadian clock. However, experiments aimed to address this issue disproved this idea. First, cocaine sensitivity is essentially the same at all times of day, and second, mutations that eliminate PDF expression show normal cocaine sensitivity (Tsai et al., 2004). These data imply that the altered cocaine sensitivity of dLmo flies is not a secondary consequence of their abnormal circadian rhythms. Moreover, while the regulation of cocaine sensitivity and circadian behaviors both localize to the pacemaker neurons, the two behaviors are genetically separable. This appears to be in contrast to findings with rodents, were several cocaine-related behaviors show diurnal rhythms that are regulated by clock genes (Abarca et al., 2002; Uz et al., 2002; Akhisaroglu et al., 2004; Kurtuncu et al., 2004; Sleipness et al., 2005; Sleipness et al., 2007).
The cocaine hypersensitivity of Lmo mutants could result from either the disruption of an LNv output that acts normally to dampen cocaine sensitivity or from an increase in an output of LNvs that normally enhances cocaine sensitivity (Fig. 4). These possibilities were differentiated by testing flies that either lack LNvs (generated by targeted expression of a cell death gene) and flies in which LNvs are selectively silenced (generated by targeted expression of tetanus toxin or a hyperpolarizing potassium channel). These genetic manipulations cause reduced sensitivity to cocaine, the opposite effect elicited by loss of dLmo function (Tsai et al., 2004), implying that dLmo acts to inhibit the contribution of LNvs to cocaine sensitivity (Fig. 4A).
Figure 4. Model for LNv and dLmo function in Drosophila.
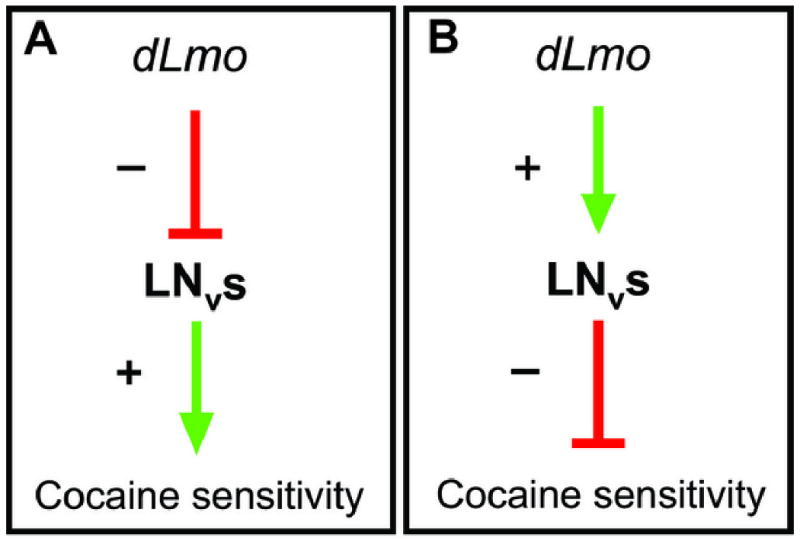
DLmo could function by (A) disrupting an LNv output that acts normally to dampen cocaine sensitivity, or (B) increasing an output of LNvs that normally enhances cocaine sensitivity. In both cases, loss of dLmo would result in enhanced cocaine sensitivity. These possibilities can be differentiated by testing flies that either lack LNvs (generated by targeted expression of a cell death gene) or flies in which LNvs are selectively silenced (generated by targeted expression of tetanus toxin or a hyperpolarizing potassium channel). These genetic manipulations cause reduced sensitivity to cocaine, the opposite effect elicited by loss of dLmo function (Tsai et al., 2004), implying that dLmo acts to inhibit the contribution of LNvs to cocaine sensitivity (panel A). (Tsai et al., 2004; reprinted with permission)
Taken together, these data show that dLmo function in PDF-expressing LNvs regulates acute sensitivity to volatilized cocaine in Drosophila. First, loss-of-function mutations in dLmo show increased cocaine sensitivity, a defect that can be reversed by induced expression of dLmo in the LNvs. Second, gain-of-function mutations in which dLmo is overexpressed, show reduced cocaine sensitivity; this resistance is mimicked by overexpression of dLmo specifically in the LNvs. The role of dLmo in cocaine sensitivity, although mapped to the circadian pacemaker neurons, is separable from its role in circadian regulation. Interestingly, expression of a mouse homolog of dLmo, Lmo4, is highly enriched in the suprachiasmatic nucleus (SCN)(AWL, DK, and UH, unpublished observations), the mammalian central pacemaker. Furthermore, microarray analysis revealed that in many mouse tissues, Lmo4 expression varies with circadian time (Panda et al., 2002). These data suggest an evolutionarily conserved role for LMOs in circadian systems, a possibility that has not yet been addressed experimentally in rodents. How do LNvs modulate cocaine responses? The observation that LNv electrical activity and synaptic output contribute to cocaine-induced behavioral responses, raises a number of possibilities regarding the interaction of cocaine with these neurons and their output. It is possible, for example, that the activity of LNvs directly increases upon cocaine administration, which in turns results in cocaine-induced changes in behavior (Fig. 5A). In agreement with this possibility is the finding that isolated cultured LNvs respond to either dopamine and acetylcholine, but not to other neurotransmitters (Wegener et al., 2004), suggesting that LNvs express dopamine receptors and may therefore be sensitive to the enhanced synaptic dopamine levels produced upon inhibition of DAT by cocaine in presynaptic neurons. However, it is also possible that other, yet to identified dopamine receptor-expressing neurons function pre- or post-synaptically to the LNvs (or in a parallel pathway) to regulate cocaine sensitivity. If dopamine receptors are expressed in LNvs, we speculate that their activity could be directly increased by cocaine administration. When the LNvs are ablated or silenced (Fig. 5B), one site of cocaine action would be eliminated, thus reducing cocaine's effect. How would Lmo fit into this model? It is possible that in dLmo loss-of-function mutants, LNv output is boosted, possibly due to increased expression of dopamine (or othere) receptors, leading to enhanced cocaine sensitivity (Fig. 5C). Conversely, increased dLmo expression in LNvs would result in reduced receptor content and, consequently, in dampened cocaine sensitivity (Fig. 5D). LMO–induced changes in receptor expression are not inconceivable given LMO's interaction with LIM–HD proteins. Changes in LIM–HD protein function have been shown to affect aspects of neuronal subtype identity, including neurotransmitter and receptor expression profiles. For example, mutations in the Drosophila LIM–HD gene islet cause a loss of dopamine and serotonin synthesis, while ectopic expression leads to ectopic expression of TH (Thor and Thomas, 1997). This graded effect of islet function on dopamine levels and TH expression is reminiscent of the graded cocaine responses observed in dLmo gain- and loss-of-function mutants. In addition, expression of a Drosophila dopamine receptor in larval neurons requires the function of the LIM-HD gene apterous (Park et al., 2004). The observation that dopamine receptor expression is also altered in various C. elegans LIM-HD mutants (Tsalik et al., 2003), suggests an evolutionarily-conserved role of LIM–HD proteins and possibly LMOs in the regulation of neurotransmitter identity and responsiveness of specific neurons. Mammalian LMOs interact with multiple transcription factors, which in turn regulate various aspects of nervous system development, including the specification of neural identity (see below) (Hobert and Westphal, 2000; Shirasaki and Pfaff, 2002). Thus, LMOs are ideally suited to modulate the neurochemical identity and sensitivity of the nervous system to various stimuli, including drugs of abuse.
Figure 5. A model for LNv and dLMO regulation of cocaine sensitivity in Drosophila.
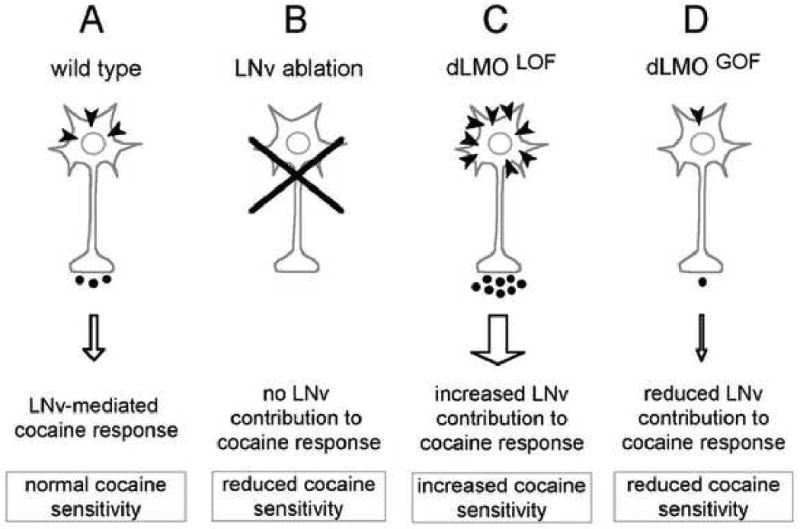
(A) In wild type, LNvs modulate locomotor responses via electrical activity and synaptic transmission. In this model, cocaine would act to directly increase LNv activity. Upon cocaine administration, synaptic dopamine concentrations would be increased (via cocaine's inhibition of the plasma membrane dopamine transporter), and activation of presumed dopamine receptors on the LNv (dark arrowheads) would stimulate electrical activity and subsequent synaptic output. This activity would then contribute to the behavioral response of the fly to cocaine. (B) LNv ablations eliminate LNv contribution to the cocaine response, reducing cocaine sensitivity. (C) dLmo loss–of–function mutants (dLmoLOF), which have increased cocaine sensitivity, would have increased activity/output during the cocaine response. This increased activity may be mediated by increases in dopamine receptor content on the LNv. (D) dLmoGOF mutants would have reduced LNv output and reduced cocaine sensitivity. This could result from a reduction in dopamine receptor density. (Tsai et al., 2004; reprinted with permission)
Role of Lmo4 in cocaine-related behaviors in mice
Mammalian genomes encode four Lmo genes, Lmo1-4, which have been studied primarily for their roles in oncogenesis. Lmo1 and 2 were first discovered as chromosomal translocations in T-cell leukemias (Rabbitts, 1998). Moreover, high expression of Lmo3 is correlated with unfavorable prognosis in neuroblastomas (Aoyama et al., 2005b), while Lmo4 plays a role in a variety of cancers (Visvader et al., 2001; Sum et al., 2005; Taniwaki et al., 2006; Murphy et al., 2008). In addition, high levels of LMO proteins contribute to increased cell proliferation, suggesting that the function of LMOs in diverse tissues is to inhibit cellular differentiation. Lmo genes are highly expressed during development, and their roles in embryogenesis have been investigated using knockout mice (Warren et al., 1994; Hahm et al., 2004; Tse et al., 2004). Mice containing a homozygous null mutation in Lmo4 die just after birth, and exhibit defective neural tube closure (Hahm et al., 2004; Tse et al., 2004; Lee et al., 2005). Clues as to the function of Lmo4 in neurons have come from cultured neuroblastoma cells, where its overexpression prevents neurite outgrowth, while its down-regulation promotes neurite formation (Vu et al., 2003), suggesting an inhibitory role in neuritogenesis. Lmo4 is widely expressed in the brain during early embryogenesis, and becomes more specifically localized during late embryogenesis, with particularly high levels in the cortex, hippocampus, subthalamic nuclei, globus pallidus, nucleus accumbens, and caudate putamen (Hermanson et al., 1999). Expression of Lmo4 in the developing mouse cerebral cortex is necessary for the organization of the barrel field in the somatosensory cortex and the proper patterning of thalamocortical connections, as demonstrated in mice with conditional knockout of Lmo4 in the cortex (Kashani et al., 2006). In addition, Lmo4 is asymmetrically expressed in the right perisylvian cerebral cortex in humans, suggesting a potential role in the development of human brain asymmetry (Sun et al., 2005). Expression of Lmo genes in the adult mouse central nervous system closely mimics expression seen during development, with high expression in the cortex, caudate putamen, amygdala, and hippocampus (Hinks et al., 1997; Hermanson et al., 1999)(see below).
As in Drosophila, mammalian LMO proteins have been shown to play a role in transcriptional regulation (Retaux and Bachy, 2002; Matthews and Visvader, 2003). LMOs interact with the mammalian homolog of Drosophila Chip, known as Ldb1 (Grutz et al., 1998; Deane et al., 2003). Ldb proteins, in turn, interact with DNA-binding LIM homeodomain (LIM-HD) proteins to regulate transcription. Large multimeric transcriptional complexes containing LMOs, Ldbs, and LIM-HD proteins are thought to specify neuronal cell fate, for example the choice between motor neuron or interneuron in the developing spinal cord (Thaler et al., 2002). In this context, high levels of LMO proteins are hypothesized to repress transcription mediated by LIM-HD factors by competing with LIM-HD factors for Ldb binding (Fig. 2). However, mammalian LMO proteins also interact with multiple other transcription factors, and can either activate or inhibit their function (Visvader et al., 1997; Wadman et al., 1997; Chan and Hong, 2001; Aoyama et al., 2005a; Singh et al., 2005; Kashani et al., 2006). The promiscuous binding of LMO proteins to many different types of transcription factors indicates that these proteins can exist in large transcriptional complexes and, depending on context, can promote transcriptional activation or repression. Generally, however, the transcriptional modifying property of LMO proteins tends to keep cells in an undifferentiated state, and in neurons appears to negatively regulate dendrite formation or axonal outgrowth. In addition, evidence showing that expression and activity of LMOs is dynamically regulated within the nervous system is growing. For instance, expression of the murine Lmo1, Lmo2, and Lmo3 genes is differentially regulated by seizure activity in specific regions of the hippocampus and forebrain of adult mice (Hinks et al., 1997). In addition, gene array experiments have revealed that expression of mouse Lmo4 is under circadian regulation in the SCN and increased in the cerebral cortex during sleep deprivation (Cirelli and Tononi, 2000; Panda et al., 2002), and Lmo3 was isolated as a transcript upregulated by dopamine administration in cultured astrocytes (Shi et al., 2001). Lastly, Lmo2 and Lmo4 were isolated in a screen for calcium–regulated activators of transcription (Aizawa et al., 2004), suggesting a role for LMOs in regulating gene expression changes induced by neural activity.
As detailed above, experiments in Drosophila revealed a role for dLmo in the acute response to cocaine, suggesting the possibility that one or more mammalian Lmo genes may mediate behavioral responses to cocaine. This possibility was tested by examining the role of Lmo4 in behavioral responses to cocaine. A mouse strain, Lmo4Gt, was generated from embryonic stem cells (Bay Genomics) that contain a gene-trap insertion in the 4th intron of Lmo4 (Lasek et al., submitted). Consistent with the reported lethality of null mutations in Lmo4, homozygous Lmo4Gt mice do not survive, indicating the generation of a null (or strong hypomorphic) allele. Lmo4Gt/+ heterozygous mice are viable and fertile with no obvious behavioral defects, despite expressing ∼50% of wild-type Lmo4 transcript levels in the brain as determined by quantitative RT-PCR (Lasek et al, submitted). The Lmo4Gt line produces an LMO4–β-galactosidase fusion protein, whose expression is regulated by endogenous Lmo4 cis-regulatory elements. Intense expression of β-galactosidase in adult Lmo4Gt/+ mice is restricted to specific brain regions, including most regions implicated in drug-related behaviors (Nestler, 2000), such as the prefrontal cortex, nucleus accumbens (Acb), and ventral tegmental area (Fig. 6A-C), data that is consistent with in situ hybridization studies (Hermanson et al., 1999). Lmo4Gt/+ mice show a modest but significant increase in the acute locomotor-stimulant and stereotypic responses to a low dose of cocaine (5 mg/kg)(Fig. 7A, B); however, no significant differences are observed upon exposure to moderate or high cocaine doses (10, 15, or 30 mg/kg). A robust enhancement in behavioral sensitization to repeated cocaine exposures is also observed in Lmo4Gt/+ mice (Lasek et al., submitted). The increase in acute cocaine sensitivity observed in heterozygous Lmo4Gt mice parallels data on dLmo and cocaine responses in Drosophila, as decreased dLmo levels lead to enhanced acute cocaine sensitivity.
Figure 6. Expression of Lmo4 in the adult mouse brain.
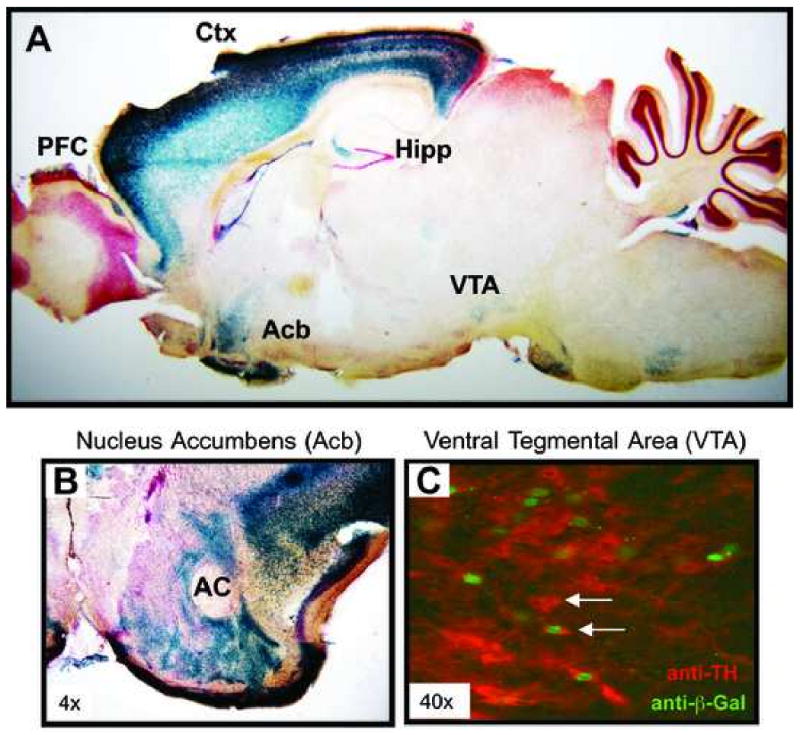
(A) Sagittal brain section of an adult Lmo4Gt/+ mouse stained with X-Gal (blue) to detect β-galactosidase expression (expressed as a Lmo4-β-galactosidase fusion protein). Intense β-galactosidase expression is observed in neocortex (Ctx; layers III and V), hippocampus (Hipp; CA3 regions and subiculum), and nucleus accumbens (Acb); low expression is also seen in the ventral tegmental area (VTA) and some hypothalamic nuclei. Expression in baso-lateral amygdala and caudate putamen is not seen in this specific sagittal section. (B) Coronal section showing β-galactosidase expression in the Acb of a Lmo4Gt/+ adult mouse. (AC = anterior commissure). (C) Expression of LMO4-β-Galactosidase fusion protein in the VTA of a Lmo4Gt/+ mouse. Brain sections containing VTA were processed for β-galactosidase (green) and tyrosine hydroxylase (TH, red) antibody reactivity. Colocalization of LMO4 fusion protein and TH is observed in some (arrow), but not all (arrowhead) TH-positive dopaminergic neurons.
Figure 7. Cocaine sensitivity phenotypes of Lmo4Gt heterozygous mice.
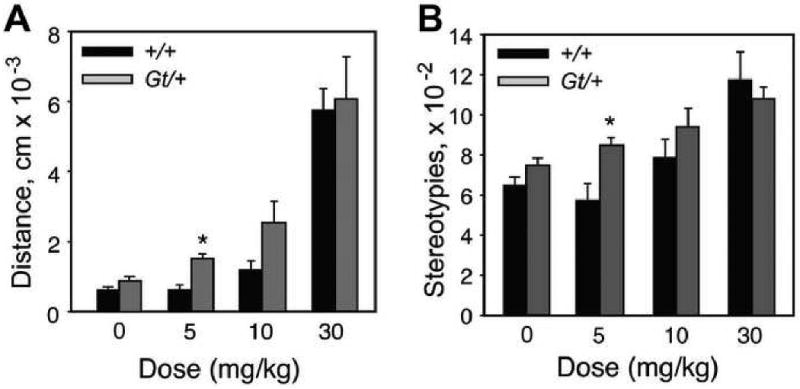
(A) Mice heterozygous for the Lmo4Gt insertion (Gt/+, gray bars) show increased locomotor activation compared to wild-type littermates (+/+, black bars) upon administration of 5 mg/kg, but not 10 nor 30 mg/kg, of cocaine. Data shows total activity for 15 minutes after injection. Asterisk indicates significant differences between groups (Student's t-test, P<0.05, n=6-13). (B) Sterotypic counts in response to acute cocaine injections. Data were analyzed as in (A).
Identification of transcriptional targets of LMO4 will likely provide relevant insights into its mechanisms of action in regulating cocaine-induced behaviors. Since dopamine and glutamate systems regulate the behavioral responses to cocaine (see, for example, Ikegami and Duvauchelle, 2004; Kalivas et al., 2005; Haile et al., 2007; Kalivas, 2007; Kalivas and O'Brien, 2008), the expression of dopamine and glutamate receptor mRNAs was examined in nucleus accumbens (Acb) tissue expressing a small hairpin RNA targeting Lmo4 (shLMO4)(Lasek et al., submitted). Significant reductions in mRNA levels for the dopamine D2 receptor (Drd2, Fig. 8A) and the GluR1 subunit of the AMPA receptor (Gria1, Fig. 8B) are observed in Acb tissue expressing shLMO4 (where Lmo4 expression is decreased by approximately 50%). Expression of dopamine D1, D3 and D5 receptors and of the GluR2 subunit of the AMPA receptor are not significantly altered upon Lmo4 downregulation (AWL, unpublished results). Interestingly, overexpression of a dominant-negative pore-dead form of GluR1 in the Acb of rats causes an enhanced acute response to cocaine (Bachtell et al., 2008). This is consistent with a predicted enhanced behavioral response to cocaine observed upon downregulation of GluR1 in the Acb of mice with decreased Lmo4 expression. In contrast, Drd2 knockout mice exhibit reduced locomotor activity and responsiveness to acute cocaine administration (Chausmer et al., 2002) The transcriptional activation of Drd2 by Lmo4 in Acb (implied by the reduced expression of Drd2 upon Lmo4 down-regulation) is therefore not consistent with the enhanced cocaine sensitivity seen upon Lmo4 downregulation. Drd2 knockout mice, however, exhibit increased cocaine self-administration at high cocaine doses, suggesting a role for Drd2 in limiting drug intake (Caine and Koob, 1994). In addition, human cocaine addicts and non-human primates chronically self-administering cocaine show decreased D2-receptor levels in striatum (Volkow et al., 1993; Nader et al., 2002), and polymorphisms of the D2 dopamine receptor resulting in reduced receptor levels have been linked to cocaine addiction (Noble et al., 1993; Persico et al., 1996). Thus, Lmo4 either directly or indirectly regulates the profile of receptors expressed by Acb neurons, an effect that likely alters the function of neural circuits that mediate behavioral responses to cocaine and possibly other abused drugs. It will be interesting to determine if the expression of Drosophila dopamine and/or glutamate receptors is regulated by dLmo and whether such potential regulation is responsible for the cocaine-sensitivity defects observed in dLmo mutant flies.
Figure 8. Altered expression of Drd2 and Gria1 upon Lmo4 downregulation in mouse Acb.
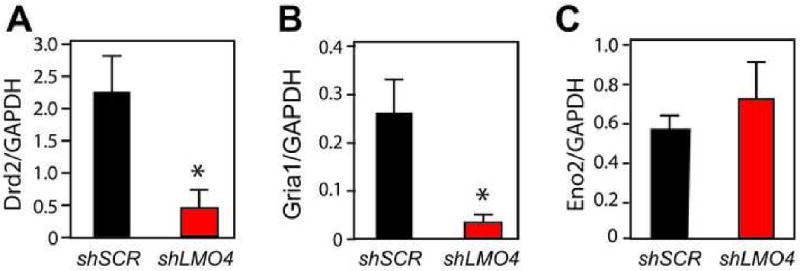
(A-C) Quantitative RT-PCR analysis of mRNA isolated from Acb infected with lentiviruses expressing either an sh-RNA that targets Lmo4 (shLMO4) or a “scrambled” control shRNA (shSCR) that is predicted to be inert. Infected cells were laser capture microdissected from brain sections taken 12 days after stereotaxic injection of virus into Acb. Expression of Drd2 (A) and Gria1 (B) are significantly different between shSCR- and shLMO4-infected samples, in contrast to the neuronal marker Eno2 (C), which shows no difference. Error represents SEM (n=5 in each group; *P<0.05 by Student's t-test).
Conclusions
It has now been well-established that drugs of abuse act in flies and mammals by similar mechanisms. Behaviors are remarkably similar, and some similarities and the molecular and neurochemical levels have already emerged. Unbiased genetic screens in Drosophila – aided by the ease, quickness, and low expense of fly studies – have been used to identify novel potential candidate genes regulating drug-related behaviors. These screens have now implicated a variety of signaling pathways and biological processes in drug-related behaviors, many of which appear to have evolutionarily conserved roles in mammals. The role of dLmo and Lmo4 in regulating cocaine-related behaviors in flies in mice, respectuvely, provides an example of such conservation. Morever, the observation that LMO4 regulates dopamine and glutamate receptor expression in the nucleus accumbens provides a possible mechanism through which LMOs may regulate cocaine-related behaviors.
Figure 1. Cocaine delivery and cocaine-induced locomotor behaviors in Drosophila.
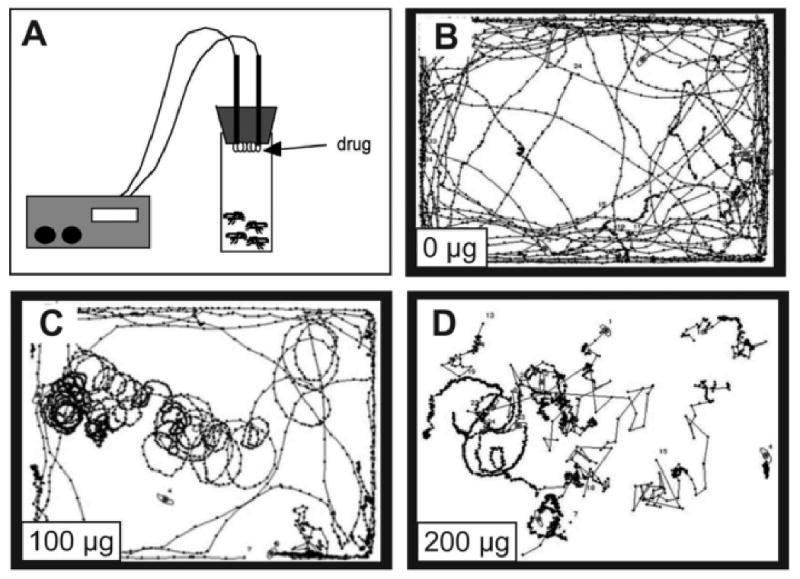
(A). Cartoon of the system used to volatilize free-base cocaine (McClung and Hirsh, 1998). Cocaine (dissolved in ethanol) is deposited on the nichrome coil and volatilized upon heating the coil. Flies “inhale” the volatilized cocaine and are then transferred to an observation chamber in which their behavior is filmed and analyzed using specialized software (Wolf et al., 2002). (B-D) Computer-generated traces of the locomotor behavior of a group of 5 flies exposed to volatilized free-base cocaine. Each panel corresponds to a one-minute period starting 2 minutes after the end of the cocaine exposure. (B) Mock exposure, (C) 100 μg cocaine, and (D) 200 μg cocaine. (Bainton, tsai, et al., 2000; reprinted with permission)
Acknowledgments
Funding was provided by grants from NIDA, the DOD, and the State of California to UH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol Biochem Behav. 2004;79:37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Aoyama M, Agari K, Sun-Wada GH, Futai M, Wada Y. Simple and straightforward construction of a mouse gene targeting vector using in vitro transposition reactions. Nucleic Acids Res. 2005a;33:e52. doi: 10.1093/nar/gni055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Ozaki T, Inuzuka H, Tomotsune D, Hirato J, Okamoto Y, Tokita H, Ohira M, Nakagawara A. LMO3 interacts with neuronal transcription factor, HEN2, and acts as an oncogene in neuroblastoma. Cancer Res. 2005b;65:4587–4597. doi: 10.1158/0008-5472.CAN-04-4630. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LTY, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Current Biology. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Bishop GB, Cullinan WE, Curran E, Gutstein HB. Abused drugs modulate RGS4 mRNA levels in rat brain: comparison between acute drug treatment and a drug challenge after chronic treatment. Neurobiol Dis. 2002;10:334–343. doi: 10.1006/nbdi.2002.0518. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- Carthew RW. Gene silencing by double-stranded RNA. Curr Opin Cell Biol. 2001;13:244–248. doi: 10.1016/s0955-0674(00)00204-0. [DOI] [PubMed] [Google Scholar]
- Chan SW, Hong W. Retinoblastoma-binding protein 2 (Rbp2) potentiates nuclear hormone receptor-mediated transcription. J Biol Chem. 2001;276:28402–28412. doi: 10.1074/jbc.M100313200. [DOI] [PubMed] [Google Scholar]
- Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 2002;163:54–61. doi: 10.1007/s00213-002-1142-y. [DOI] [PubMed] [Google Scholar]
- Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, Deshpande SN, B KT, Ferrell RE, Middleton FA, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11:1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- Deane JE, Mackay JP, Kwan AH, Sum EY, Visvader JE, Matthews JM. Structural basis for the recognition of ldb1 by the N-terminal LIM domains of LMO2 and LMO4. Embo J. 2003;22:2224–2233. doi: 10.1093/emboj/cdg196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo J, Tully T. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends in Neurosciences. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic N, Dzitoyeva S, Manev H. An automated assay of the behavioral effects of cocaine injections in adult Drosophila. J Neurosci Methods. 2004;137:181–184. doi: 10.1016/j.jneumeth.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Engels WR. The P family of transposable elements in Drosophila. Annual Review of Genetics. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fernandez-Funez P, Lu CH, Rincon-Limas DE, Garcia-Bellido A, Botas J. The relative expression amounts of apterous and its co-factor dLdb/Chip are critical for dorso-ventral compartmentalization in the Drosophila wing. Embo J. 1998;17:6846–6853. doi: 10.1093/emboj/17.23.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Geracitano R, Federici M, Prisco S, Bernardi G, Mercuri NB. Inhibitory effects of trace amines on rat midbrain dopaminergic neurons. Neuropharmacology. 2004;46:807–814. doi: 10.1016/j.neuropharm.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Han MH, Herman AE, Ni YG, Pudiak CM, Aghajanian GK, Liu RJ, Potts BW, Mumby SM, Nestler EJ. Regulation of RGS proteins by chronic morphine in rat locus coeruleus. Eur J Neurosci. 2003;17:971–980. doi: 10.1046/j.1460-9568.2003.02529.x. [DOI] [PubMed] [Google Scholar]
- Granderath S, Stollewerk A, Greig S, Goodman CS, O'Kane CJ, Klambt C. loco encodes an RGS protein required for Drosophila glial differentiation. Development. 1999;126:1781–1791. doi: 10.1242/dev.126.8.1781. [DOI] [PubMed] [Google Scholar]
- Grillet N, Pattyn A, Contet C, Kieffer BL, Goridis C, Brunet JF. Generation and characterization of Rgs4 mutant mice. Mol Cell Biol. 2005;25:4221–4228. doi: 10.1128/MCB.25.10.4221-4228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutz G, Forster A, Rabbitts TH. Identification of the LMO4 gene encoding an interaction partner of the LIM-binding protein LDB1/NLI1: a candidate for displacement by LMO proteins in T cell acute leukaemia. Oncogene. 1998;17:2799–2803. doi: 10.1038/sj.onc.1202502. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Hahm K, Sum EY, Fujiwara Y, Lindeman GJ, Visvader JE, Orkin SH. Defective neural tube closure and anteroposterior patterning in mice lacking the LIM protein LMO4 or its interacting partner Deaf-1. Mol Cell Biol. 2004;24:2074–2082. doi: 10.1128/MCB.24.5.2074-2082.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Genetics of dopamine and its contribution to cocaine addiction. Behav Genet. 2007;37:119–145. doi: 10.1007/s10519-006-9115-2. [DOI] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hall JC. Genetics of biological rhythms in drosophila. Advances in Genetics. 1998;38:135–184. doi: 10.1016/s0065-2660(08)60143-1. [DOI] [PubMed] [Google Scholar]
- Hardie SL, Zhang JX, Hirsh J. Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev Neurobiol. 2007;67:1396–1405. doi: 10.1002/dneu.20459. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Sugihara TM, Andersen B. Expression of LMO-4 in the central nervous system of the embryonic and adult mouse. Cell Mol Biol (Noisy-le-grand) 1999;45:677–686. [PubMed] [Google Scholar]
- Hinks GL, Shah B, French SJ, Campos LS, Staley K, Hughes J, Sofroniew MV. Expression of LIM protein genes Lmo1, Lmo2, and Lmo3 in adult mouse hippocampus and other forebrain regions: differential regulation by seizure activity. J Neurosci. 1997;17:5549–5559. doi: 10.1523/JNEUROSCI.17-14-05549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Westphal H. Functions of LIM-homeobox genes. Trends Genet. 2000;16:75–83. doi: 10.1016/s0168-9525(99)01883-1. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Duvauchelle CL. Dopamine mechanisms and cocaine reward. Int Rev Neurobiol. 2004;62:45–94. doi: 10.1016/S0074-7742(04)62002-2. [DOI] [PubMed] [Google Scholar]
- Kalidas S, Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Qiu Z, Jurata L, Lee SK, Pfaff S, Goebbels S, Nave KA, Ghosh A. Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J Neurosci. 2006;26:8398–8408. doi: 10.1523/JNEUROSCI.0618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–205. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Laakso A, Mohn AR, Gainetdinov RR, Caron MG. Experimental genetic approaches to addiction. Neuron. 2002;36:213–228. doi: 10.1016/s0896-6273(02)00972-8. [DOI] [PubMed] [Google Scholar]
- Lee SK, Jurata LW, Nowak R, Lettieri K, Kenny DA, Pfaff SL, Gill GN. The LIM domain-only protein LMO4 is required for neural tube closure. Mol Cell Neurosci. 2005;28:205–214. doi: 10.1016/j.mcn.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Lindsley DL. The Genome of Drosophila Melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Verstreken P, Ostrin EJ, Phillippi A, Lichtarge O, Bellen HJ. A Genome-Wide Search for Synaptic Vesicle Proteins in Drosophila. Neuron. 2000;26:45–50. doi: 10.1016/s0896-6273(00)81136-8. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Matthews JM, Visvader JE. LIM-domain-binding protein 1: a multifunctional cofactor that interacts with diverse proteins. EMBO Rep. 2003;4:1132–1137. doi: 10.1038/sj.embor.7400030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Current Biology. 1998;8:109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Current Biology. 1999;9:853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004:pl6. doi: 10.1126/stke.2202004pl6. 2004. [DOI] [PubMed] [Google Scholar]
- Meyer G, Feldman EL. Signaling mechanisms that regulate actin-based motility processes in the nervous system. J Neurochem. 2002;83:490–503. doi: 10.1046/j.1471-4159.2002.01185.x. [DOI] [PubMed] [Google Scholar]
- Milan M, Cohen SM. Regulation of LIM homeodomain activity in vivo: a tetramer of dLDB and apterous confers activity and capacity for regulation by dLMO. Mol Cell. 1999;4:267–273. doi: 10.1016/s1097-2765(00)80374-3. [DOI] [PubMed] [Google Scholar]
- Milan M, Diaz-Benjumea FJ, Cohen SM. Beadex encodes an LMO protein that regulates Apterous LIM-homeodomain activity in Drosophila wing development: a model for LMO oncogene function. Genes Dev. 1998;12:2912–2920. doi: 10.1101/gad.12.18.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, Bahn M, Johnson R, Madras BK. Primate trace amine receptor 1 modulation by the dopamine transporter. J Pharmacol Exp Ther. 2005;313:983–994. doi: 10.1124/jpet.105.084459. [DOI] [PubMed] [Google Scholar]
- Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microscopy Research and Technique. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Murphy NC, Scarlett CJ, Kench JG, Sum EY, Segara D, Colvin EK, Susanto J, Cosman PH, Lee CS, Musgrove EA, et al. Expression of LMO4 and outcome in pancreatic ductal adenocarcinoma. Br J Cancer. 2008;98:537–541. doi: 10.1038/sj.bjc.6604177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EW, Sutton GG, Delcher AL, Dew IM, Fasulo DP, Flanigan MJ, Kravitz SA, Mobarry CM, Reinert KH, Remington KA, et al. A whole-genome assembly of Drosophila. Science. 2000;287:2196–2204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Genes and addiction. Nat Genet. 2000;26:277–281. doi: 10.1038/81570. taf/DynaPage.taf?file=/ng/journal/v226/n273/full/ng1100_1277.html taf/DynaPage.taf?file=/ng/journal/v1126/n1103/abs/ng1100_1277.html. [DOI] [PubMed]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Blum K, Khalsa ME, Ritchie T, Montgomery A, Wood RC, Fitch RJ, Ozkaragoz T, Sheridan PJ, Anglin MD, et al. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 1993;33:271–285. doi: 10.1016/0376-8716(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Park D, Han M, Kim YC, Han KA, Taghert PH. Ap-let neurons--a peptidergic circuit potentially controlling ecdysial behavior in Drosophila. Dev Biol. 2004;269:95–108. doi: 10.1016/j.ydbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bird G, Gabbay FH, Uhl GR. D2 dopamine receptor gene TaqI A1 and B1 restriction fragment length polymorphisms: enhanced frequencies in psychostimulant-preferring polysubstance abusers. Biol Psychiatry. 1996;40:776–784. doi: 10.1016/0006-3223(95)00483-1. [DOI] [PubMed] [Google Scholar]
- Rabbitts TH. LMO T-cell translocation oncogenes typify genes activated by chromosomal translocations that alter transcription and developmental processes. Genes Dev. 1998;12:2651–2657. doi: 10.1101/gad.12.17.2651. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Retaux S, Bachy I. A short history of LIM domains (1993-2002): from protein interaction to degradation. Mol Neurobiol. 2002;26:269–281. doi: 10.1385/MN:26:2-3:269. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila [see comments] Science. 2000;288:2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- Ropers HH, Hamel BC. X-linked mental retardation. Nat Rev Genet. 2005;6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Heberlein U. Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Curr Opin Neurobiol. 2002;12:639–645. doi: 10.1016/s0959-4388(02)00380-x. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Lewis EB. A brief history of Drosophila's contributions to genome research. Science. 2000;287:2216–2218. doi: 10.1126/science.287.5461.2216. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, et al. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57:543–550. doi: 10.1016/s0091-3057(96)00447-9. [DOI] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Gold SJ, McGinty JF. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. J Neurochem. 2006;96:1606–1615. doi: 10.1111/j.1471-4159.2006.03669.x. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Hearing MC, See RE, McGinty JF. Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport. 2007;18:1261–1265. doi: 10.1097/WNR.0b013e328240507a. [DOI] [PubMed] [Google Scholar]
- Shi J, Cai W, Chen X, Ying K, Zhang K, Xie Y. Identification of dopamine responsive mRNAs in glial cells by suppression subtractive hybridization. Brain Res. 2001;910:29–37. doi: 10.1016/s0006-8993(01)02393-9. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shoresh M, Orgad S, Shmueli O, Werczberger R, Gelbaum D, Abiri S, Segal D. Overexpression Beadex mutations and loss-of-function heldup-a mutations in Drosophila affect the 3′ regulatory and coding components, respectively, of the Dlmo gene. Genetics. 1998;150:283–299. doi: 10.1093/genetics/150.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR, Barnes CJ, Talukder AH, Fuqua SA, Kumar R. Negative regulation of estrogen receptor alpha transactivation functions by LIM domain only 4 protein. Cancer Res. 2005;65:10594–10601. doi: 10.1158/0008-5472.CAN-05-2268. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Res. 2005;1065:132–137. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiol Behav. 2007;91:523–530. doi: 10.1016/j.physbeh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB. Drosophila: genetics meets behaviour. Nat Rev Genet. 2001;2:879–890. doi: 10.1038/35098592. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Stebbins MJ, Urlinger S, Byrne G, Bello B, Hillen W, Yin JC. Tetracycline-inducible systems for Drosophila. Proc Natl Acad Sci U S A. 2001;98:10775–10780. doi: 10.1073/pnas.121186498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins MJ, Yin JC. Adaptable doxycycline-regulated gene expression systems for Drosophila. Gene. 2001;270:103–111. doi: 10.1016/s0378-1119(01)00447-4. [DOI] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Sum EY, Segara D, Duscio B, Bath ML, Field AS, Sutherland RL, Lindeman GJ, Visvader JE. Overexpression of LMO4 induces mammary hyperplasia, promotes cell invasion, and is a predictor of poor outcome in breast cancer. Proc Natl Acad Sci U S A. 2005;102:7659–7664. doi: 10.1073/pnas.0502990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, Orkin SH, Geschwind DH, Walsh CA. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZY, Wei J, Xie L, Shen Y, Liu SZ, Ju GZ, Shi JP, Yu YQ, Zhang X, Xu Q, Hemmings GP. The CLDN5 locus may be involved in the vulnerability to schizophrenia. Eur Psychiatry. 2004;19:354–357. doi: 10.1016/j.eurpsy.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Taniwaki M, Daigo Y, Ishikawa N, Takano A, Tsunoda T, Yasui W, Inai K, Kohno N, Nakamura Y. Gene expression profiles of small-cell lung cancers: molecular signatures of lung cancer. Int J Oncol. 2006;29:567–575. [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Thor S, Thomas JB. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron. 1997;18:397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres G, Horowitz JM. Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogaster. Synapse. 1998;29:148–161. doi: 10.1002/(SICI)1098-2396(199806)29:2<148::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tsai LT, Bainton RJ, Blau J, Heberlein U. Lmo mutants reveal a novel role for circadian pacemaker neurons in cocaine-induced behaviors. PLoS Biol. 2004;2:e408. doi: 10.1371/journal.pbio.0020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse E, Smith AJ, Hunt S, Lavenir I, Forster A, Warren AJ, Grutz G, Foroni L, Carlton MB, Colledge WH, et al. Null mutation of the Lmo4 gene or a combined null mutation of the Lmo1/Lmo3 genes causes perinatal lethality, and Lmo4 controls neural tube development in mice. Mol Cell Biol. 2004;24:2063–2073. doi: 10.1128/MCB.24.5.2063-2073.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- van Meyel DJ, O'Keefe DD, Jurata LW, Thor S, Gill GN, Thomas JB. Chip and apterous physically interact to form a functional complex during Drosophila development. Mol Cell. 1999;4:259–265. doi: 10.1016/s1097-2765(00)80373-1. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Mao X, Fujiwara Y, Hahm K, Orkin SH. The LIM-domain binding protein Ldb1 and its partner LMO2 act as negative regulators of erythroid differentiation. Proc Natl Acad Sci U S A. 1997;94:13707–13712. doi: 10.1073/pnas.94.25.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Venter D, Hahm K, Santamaria M, Sum EY, O'Reilly L, White D, Williams R, Armes J, Lindeman GJ. The LIM domain gene LMO4 inhibits differentiation of mammary epithelial cells in vitro and is overexpressed in breast cancer. Proc Natl Acad Sci U S A. 2001;98:14452–14457. doi: 10.1073/pnas.251547698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Vu D, Marin P, Walzer C, Cathieni MM, Bianchi EN, Saidji F, Leuba G, Bouras C, Savioz A. Transcription regulator LMO4 interferes with neuritogenesis in human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2003;115:93–103. doi: 10.1016/s0169-328x(03)00119-0. [DOI] [PubMed] [Google Scholar]
- Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. Embo J. 1997;16:3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Wegener C, Hamasaka Y, Nassel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol. 2004;91:912–923. doi: 10.1152/jn.00678.2003. [DOI] [PubMed] [Google Scholar]
- Weihe U, Milan M, Cohen SM. Regulation of Apterous activity in Drosophila wing development. Development. 2001;128:4615–4622. doi: 10.1242/dev.128.22.4615. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2003;54:161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci USA. 1998;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T. Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol. 2000;10:790–795. doi: 10.1016/s0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Zeng C, Justice NJ, Abdelilah S, Chan YM, Jan LY, Jan YN. The Drosophila LIM-only gene, dLMO, is mutated in Beadex alleles and might represent an evolutionarily conserved function in appendage development. Proc Natl Acad Sci U S A. 1998;95:10637–10642. doi: 10.1073/pnas.95.18.10637. [DOI] [PMC free article] [PubMed] [Google Scholar]


