Abstract
The orexins (or hypocretins) are hypothalamic neuropeptides that have been implicated in a variety of behaviors ranging from feeding to sleep and arousal. Evidence from animal models suggests a role for orexins in reward processing and drug addiction. In this review, we discuss orexin’s interaction with the mesocorticolimbic reward pathway and the effects of drugs of abuse on the orexin system. We further review models of drug dependence and addiction and describe behavioral alterations that are seen when the orexin system is manipulated both pharmacologically and genetically. Based on the findings reported in the literature thus far, we posit that orexin functioning contributes to both drug reward and drug related stress/aversive responsiveness; however, diverse anatomical substrates, and perhaps receptor specificity, contribute differentially to reward and stress components.
1. Orexin-Drug Interactions
1.1 Orexin Background
The neuropeptides orexins (or hypocretins) have been implicated in a variety of behavioral states ranging from feeding (Sakurai et al., 1998) to sleep and arousal (Chemelli et al., 1999). The orexins are produced in hypothalamic neurons restricted to the dorsal medial hypothalamus (DMH), perifornical area (PFA), and lateral regions of the lateral hypothalamus (LLH) (Date et al., 1999; De Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998). Orexin-releasing neurons also release dynorphin (Chou et al., 2001) and are intermingled, but are not co-localized, with MCH-releasing neurons (Broberger et al., 1998; Elias et al., 1998; Peyron et al., 1998). The orexin ligands, orexin A and orexin B, arise from the precursor peptide prepro-orexin by proteolytic processing (De Lecea et al., 1998; Sakurai et al., 1998). The two orexin receptors are G-protein coupled and differ in their selectivity to the orexin ligands: the orexin 1 receptor (Ox1r) is selective for orexin A whereas the orexin 2 receptor (Ox2r) is nonselective (Sakurai et al., 1998). The Ox1r is Gq-coupled and the Ox2r is Gq and Gi/o-coupled (Sakurai et al., 1998; van den Pol et al., 1998) and both receptors have been found on pre- and post-synaptic processes as well as on soma at the level of the medial and lateral hypothalamic areas (van den Pol et al., 1998). The orexin receptors (Lu et al., 2000; Marcus et al., 2001; Trivedi et al., 1998) and orexinergic projections from the lateral hypothalamic region (LH) (Mondal et al., 1999; Nambu et al., 1999) can be found throughout the brain, including brain regions known for their involvement in drug reward and addiction.
1.2 Orexin and the mesocorticolimbic reward pathway (See Figure 1)
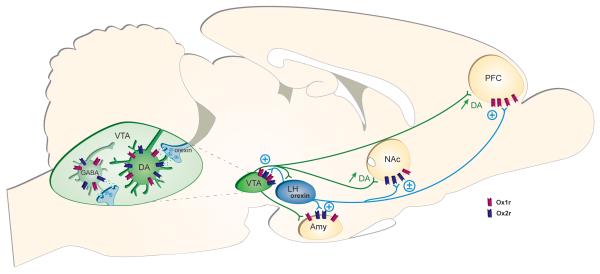
Figure 1.
Schematic representation of the orexin system and its interactions with the mesocorticolimbic reward pathway. Orexin projections (blue arrows) activate the ventral tegmental area (VTA), amygdala (Amy), and prefrontal cortex (PFC). Conflicting data suggest both activation and inhibition of the nucleus accumbens (NAc) by orexin. In the VTA, orexin activates both dopamine and GABA neurons. Orexin’s activation of the VTA results in increased dopamine (DA) in via projections (green arrows) to the NAc and PFC. Orexin’s action in the PFC is due to stimulation of Ox1rs. Orexin’s action in the NAc and amygdala is due to stimulation of Ox2rs.
Early studies showed broad projections of orexin neurons including to the midbrain dopamine (DA) neurons of the ventral tegmental area (VTA) (Baldo et al., 2003; Fadel and Deutch, 2002; Peyron et al., 1998). Studies of drug reward and drug-induced neuroadaptations have focused on orexin’s effects on the VTA and the mesocorticolimbic target regions, such as the nucleus accumbens (NAc) and amygdala as well as medial prefrontal cortex (mPFC) (Fallon and Moore, 1978; Lindvall and Bjorklund, 1974; Ungerstedt, 1971). Microdialysis studies have demonstrated that most, if not all, abused drugs increase extracellular DA levels at the level of the NAc (Carboni et al., 1989; Di Chiara and Imperato, 1988; Imperato and Di Chiara, 1986; Imperato et al., 1986), and neuroadaptations in this system is thought to underlie many elements of addiction as reviewed in (Koob et al., 1998). The hypothalamic orexin system is positioned to interact with the mesocorticolimbic pathway via many brain regions, but most work has focused on the VTA.
Orexin projections from the LH are localized in the VTA (Baldo et al., 2003; Fadel and Deutch, 2002; Nakamura et al., 2000; Peyron et al., 1998) and orexin terminals contact tyrosine-hydroxylase (TH) positive cells (Fadel and Deutch, 2002; Nakamura et al., 2000). Although it has been reported that orexin axons infrequently synapse onto dopamine and GABA neurons, the VTA does contain numerous orexin-containing dense core vesicles suggesting nonsynaptic effects (Balcita-Pedicino and Sesack, 2007). Both orexin receptors have been found to be present in high density in the VTA (Lu et al., 2000; Narita et al., 2006) on both DA-containing (Korotkova et al., 2003; Narita et al., 2006) and GABA-containing neurons (Korotkova et al., 2003). Orexin exerts an excitatory action in the VTA and activates both DA (Korotkova et al., 2003; Nakamura et al., 2000) and non-DA cells (Korotkova et al., 2003) via a direct postsynaptic effect. Intra-VTA application of orexin results in increased Fos expression in DA neurons located specifically in the caudomedial portion of the VTA (Vittoz et al., 2008) and increases DA at the level of the NAc shell, but not in the NAc core (Narita et al., 2006; Vittoz and Berridge, 2006) and the mPFC (Narita et al., 2006; Vittoz and Berridge, 2006). Intra-VTA orexin also results in potentiation of NMDA receptor-mediated excitatory postsynaptic currents (Borgland et al., 2006) indicating a role for orexin in long term neural plasticity.
Orexin fibers have also been reported in the limbic structures terminating the mesocorticolimbic pathway (Fadel and Deutch, 2002; Marcus et al., 2001; Peyron et al., 1998). Although both Ox1r and Ox2r have been reported to be expressed in the NAc (Martin et al., 2002), Ox1r levels are very low (Marcus et al., 2001) and orexin’s actions have been attributed to Ox2r binding (Martin et al., 2002). Unlike the excitatory effects of orexin in the VTA, activation of orexin receptors in the NAc has been reported to result in inhibition (Martin et al., 2002). However, a more recent finding reports that orexin application depolarizes NAc shell neurons via Ox2r (Mukai et al., 2009). Different experimental methodologies may explain these conflicting findings or perhaps Ox2r activation has both excitatory and inhibitory postsynaptic effects. Further investigation is necessary to clarify orexin’s effects in the NAc. Orexin action in the amygdala is excitatory and is also mediated by Ox2r (Bisetti et al., 2006); however both orexin receptors have been reported in the amygdala (Marcus et al., 2001). Orexin is also excitatory in the mPFC via activation of the Ox1r (Xia et al., 2005) and the Ox1r is the only orexin receptor reported in the mPFC (Marcus et al., 2001; Trivedi et al., 1998).
1.3. Direct drug action on orexin system (See Figure 2)
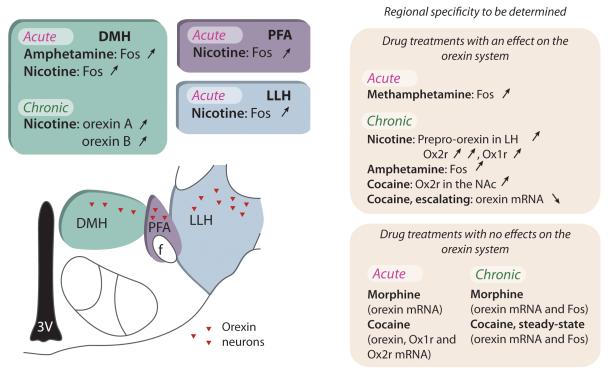
Figure 2.
Effects of acute or chronic drug adminsitration on orexin cells in the LH. Abbr: DMH-dorsal medial hypothalamus; PFA-perifornical area; LLH-lateral portion of lateral hypothalamus; 3V-third ventricle; f-fornix. Data derived from (Estabrooke et al., 2001; Fadel and Deutch, 2002; Georgescu et al., 2003; Kane et al., 2000; Morshedi and Meredith, 2008; Pasumarthi et al., 2006; Pasumarthi and Fadel, 2008; Sharf et al., 2008; Zhang et al., 2007; Zhou et al., 2006; Zhou et al., 2008)
Drugs of abuse have direct effects on the orexin system and these effects are drug-specific. Moreover, regional variations within the LH have been reported in response to some drugs. For instance, Fos expression is increased in orexin neurons following acute administration of methamphetamine (Estabrooke et al., 2001), amphetamine (Fadel and Deutch, 2002), and nicotine (Pasumarthi et al., 2006; Pasumarthi and Fadel, 2008). In response to some drugs, such as nicotine, Fos expression increases in orexin neurons in both the lateral and medial portions of the LH (Pasumarthi et al., 2006). However, amphetamine increases Fos expression in neurons specifically in the DMH, but not in PFA or LLH (Fadel and Deutch, 2002). On the contrary, acute administration of cocaine fails to affect either of the orexin receptors (Zhang et al., 2007; Zhou et al., 2008) or peptide levels (Zhang et al., 2007; Zhou et al., 2008) and acute morphine fails to alter orexin mRNA levels (Zhou et al., 2006).
In addition to effects of acute drugs, chronic drugs of abuse also influence the orexin system via changes in orexin neural activation and mRNA levels of orexin or its receptors. Chronic administration of nicotine is associated with increased expression of prepro-orexin mRNA in the LH (Kane et al., 2000). Orexin A expression is increased primarily in the DMH whereas orexin B is increased in DMH and paraventricular hypothalamus (PVN) (Kane et al., 2000). Furthermore, chronic nicotine is associated with increases of Ox2r mRNA and, to a lesser degree, increases of Ox1r mRNA (Kane et al., 2000). Chronic amphetamine exposure results in increased Fos expression in orexin neurons (Morshedi and Meredith, 2008). In contrast, chronic morphine treatment (Georgescu et al., 2003; Sharf et al., 2008; Zhou et al., 2006) and steady-state chronic cocaine (Zhou et al., 2008) fail to affect orexin mRNA levels or Fos expression in orexin neurons in the LH. Nonetheless, Ox2r levels are increased in the NAc following chronic cocaine; a change that persists even months following cessation of cocaine treatment (Zhang et al., 2007). Although orexin mRNA levels are unchanged in response to steady-state cocaine, chronic escalating dose cocaine treatment results in decreased orexin mRNA levels in the LH (Zhou et al., 2008). It should also be recognized that negative results with gene expression analysis could be a function of the timepoint chosen for analysis.
2. Behavioral investigation of orexin’s role in drug dependence and addiction (See Table 1)
Table 1.
Summary of behavioral data supporting a role for orexin in responses to drugs of abuse and food.
| Acute Hyperlocomotion | ||
| Cocaine | SB-334867 has no effect | (Borgland et al 2006) |
| Morphine | OKO show reduction | (Narita et al 2006) |
| SB-332867 has no effect | (Sharf et al Submitted) | |
| OKO show normal behavior | (Sharf et al Submitted) | |
| Sensitization | ||
| Cocaine | SB-334867 blocks development not expression | (Borgland et al 2006) |
| Morphine | SB-334867 has no effect | (Sharf et al Submitted) |
| OKO show normal behavior | (Sharf et al Submitted) | |
| Withdrawal | ||
| Cocaine | No activation of orexin neurons | (Zhou et al 2008) |
| Morphine | Increases CRE in orexin cells | (Georgescu et al 2008) |
| Increases Fos in orexin cells | (Sharf et al 2008) | |
| Increases orexin mRNA in LH | (Zhou et al 2006) | |
| OKO show reduced withdrawal symptoms | (Georgescu et al 2008) | |
| SB-334867 attenuates withdrawal symptoms | (Sharf et al 2008) | |
| Self-administration | ||
| Cocaine | SB-334867 or orexin no effect | (Boutrel et al., 2005; Aston-Jones et al., 2009) |
| Nicotine | SB-334867 reduces | (Hollander et al 2008) |
| Ethanol | SB-338467 reduces | (Lawrence et al 2006) |
| Orexin A increases | (Schneider et al 2007) | |
| Food | SB-334867 reduces | (Nair et al., 2008; Thorpe and Kotz, 2005) |
| Self-administration extinction | ||
| Cocaine | SB-334867 reduces extinction responding | (Aston-Jones 2009) |
| Self-administration reinstatement | ||
| Cocaine | Orexin A reinstates | (Boutrel et al 2005; Wang et al 2009) |
| SB-334867 attenuates cue-primed reinstatement | (Aston-Jones 2009) | |
| Ethanol | SB-334867 attenuates cue-primed reinstatement | (Lawrence et al 2006) |
| Fos increases in orexin cells following CS presentations | (Dayas et al 2008) | |
| Food | Orexin A reinstates | (Boutrel et al 2005) |
| Conditioned Place preference | ||
| Cocaine | Increases Fos in orexin neurons | (Harris et al 2005) |
| Decreases orexin mRNA in LLH | (Zhou et al 2008) | |
| SB-334867 has no effect | (Sharf et al Submitted) | |
| Morphine | Increases Fos in orexin neurons | (Harris et al 2005) |
| SB-334867 reduces CPP | (Harris et al 2005; US; Narita et al 2006) | |
| OKO show no CPP | (Narita et al 2006) | |
| OKO show normal behavior | (Sharf et al Submitted) | |
| Food | Increases Fos in orexin neurons | (Harris et al 2005) |
2.1 Locomotor Hyperactivity and Sensitization
Drug-induced hyperlocomotion and behavioral sensitization is argued to represent a form of drug induced neural plasticity (Robinson et al., 1982) that may contribute to dependence and addictive processes. To assess orexin’s involvement in drug dependence, manipulation of the orexin system via blockade of Ox1rs by the administration of SB-334867, has been proven to be a useful strategy. SB-334867 is a selective Ox1r antagonist with 50 fold selectivity for Ox1r over Ox2r (Duxon et al., 2001). In addition, orexin -/- mice (OKO), who lack the gene for prepro-orexin, have been useful in the investigation of orexin-drug interactions. Thus far, investigation of orexin’s role in drug hyperlocomotion and sensitization has been limited. Systemic adminsitration of SB-334867 does not affect acute cocaine-induced hyperlocomotion, but systemic and intra-VTA blockade of Ox1r blocks the development of cocaine sensitization. SB-334867 fails to affect locomotor activity in already cocaine-sensitized animals (Borgland et al., 2006). Furthermore, we have recently found that SB-334867 treated mice and OKO mice respond normally following an acute morphine treatment and no changes in behavioral sensitization are seen following chronic morphine administration (Sharf et al., Submitted). In contrast, previous data does suggest that OKO mice show reduced hyperlocomotion in response to acute morphine and that OKO mice do not differ from wild-type (WT) controls in basal DA levels in the NAc, but DA increases in response to morphine were greater in WT mice (Narita et al., 2006). These limited and conflicting findings suggest that more investigation into the role of orexin in locomotor responses to drugs of abuse is needed.
2.2 Withdrawal
Withdrawal from abused drugs can also alter the orexin system, and the first study connecting orexin to drugs of abuse demonstrated the involvement of orexin in morphine withdrawal (Georgescu et al., 2003). Naloxone- or naltrexone precipitated morphine withdrawal leads to the induction of CRE activity in orexin neurons (Georgescu et al., 2003) and Fos expression in orexin cells (Georgescu et al., 2003; Sharf et al., 2008). Interestingly, increases in Fos expression were restricted to the DMH and PFA, and were not seen in the LLH (Sharf et al., 2008). Spontaneous morphine withdrawal increases orexin mRNA in the LH (Zhou et al., 2006). Activation of orexin neurons in response to withdrawal appears to be opiate specific and is not seen following spontaneous withdrawal following chronic cocaine (Zhou et al., 2008).
Manipulations of the orexin system can affect behavioral responses seen during withdrawal. For instance, naltrexone precipitated morphine withdrawal is attenuated in OKO mice (Georgescu et al., 2003). Similarly, SB-334867 treatment prior to naloxone-precipitated withdrawal attenuates somatic withdrawal symptoms (Sharf et al., 2008). The degree of somatic withdrawal symptoms is correlated with cFos expression in the NAc shell and blockade of Ox1r indirectly attenuates NAc cellular activation (Sharf et al., 2008). Interestingly, the VTA does not show changes in cFos expression in the same animals, suggesting a possible non-VTA mechanism by which orexin alters NAc neuronal activity.
2.3 Self-administration, Extinction, and Reinstatement
Most behavioral measures of reward assess the hedonic, or rewarding, effects of stimuli indirectly. Of the different tests performed, self-administration studies are powerful measures that best mimic conditions of addiction whereby the subject is controlling the drug delivery and drug seeking behavior. The role of orexin in drug reward, using self-administration paradigms, has been reported. SB-334867 administration results in decreased self-administration of nicotine (Hollander et al., 2008) and alcohol (Lawrence et al., 2006) and intra-LH orexin A increases alcohol intake (Schneider et al., 2007). However, these results are not seen with all drugs of abuse as intracerebroventricular (icv) administration of orexin A (Boutrel et al., 2005) or treatment with SB-334867 (Aston-Jones et al., 2009) fails to affect responding during active cocaine self-adminsitration.
The majority of the literature thus far has focused on the role of orexin in extinction and reinstatement of an extinguished response. Here, following training, animals continue to lever-press or nose-poke, but no drug is delivered following previously correct responses until eventually animals cease to respond at high levels. On the first day of extinction training, animals typically display a burst of activity characterized by a particularly large number of responses despite the lack of drug infusion. Interestingly, administration of SB-334867 prior to the first day of extinction in animals previously trained to self-administer cocaine eliminates the extinction burst response (Aston-Jones et al., 2009). Once extinguished, manipulation of the orexin system can directly lead to reinstatement of extinguished responses. For instance, icv administration of orexin A reinstates extinguished responses in animals trained to self-administer cocaine and food reinforcers (Boutrel et al., 2005). Intra-VTA administration of orexin A also reinstates extinguished cocaine-seeking (Wang et al., 2009).
Reinstatement of the extinguished response can be induced by presentation of various stimuli, including the drug (drug-primed reinstatement) or cues paired with the previously active response (cue-primed reinstatement). Treatment with SB-334867 fails to affect cocaine-primed reinstatement, but attenuates cue-primed cocaine reinstatement (Aston-Jones et al., 2009). Similarly, the presentation of alcohol-associated cues reinstates extinguished alcohol self-administration, but not in animals treated with SB-334867 (Lawrence et al., 2006). Furthermore, the presentation of alcohol-paired cues during reinstatement is accompanied by increased Fos expression in orexin neurons (Dayas et al., 2008) and the degree of alcohol seeking is positively correlated with Fos expression in the LLH in both orexin-positive and orexin-negative neurons (Hamlin et al., 2007).
The reinstatement findings summarized above suggest that activation of the orexin system can result in spontaneous reinstatement of a previously extinguished response. Moreover, orexin functioning is involved in cue-primed, but not drug-primed, reinstatement. It is possible that orexin plays a more general role in mediating cue- and/or context-drug associations.
2.4 Conditioned Place Preference
The conditioned place preference (CPP) test has become a widely-used paradigm for the measure of reward-related behavior. Here, an animal, is placed in an enclosed environment, which is divided into two or more distinct compartment, each containing distinctive environmental cues that differentiate it from the other(s). After the animal is allowed to move freely among the compartments, it is confined to one of the compartments and is presented with a rewarding stimulus. The animal forms an association between the rewarding stimulus and the environmental cues during this time. After the pairing, when allowed to roam freely among the compartments, the animal will spend more time in the reward-paired chamber if the drug was rewarding. Although CPP is often used to assess the rewarding effects of drugs of abuse, animals are tested in a drug-free state and preference is indicative of the rewarding effects of the context rather than of the drug directly as reviewed in (Bardo and Bevins, 2000). Furthermore, some discrepancy has been reported between CPP data and self-administration data (Bardo et al., 1999; Deroche et al., 1999) suggesting that these paradigms may be investigating different components of drug reward and addiction. Nonetheless, CPP remains a prominent and useful experimental protocol in the investigation of neural mechanisms underling drug addiction.
It has been shown that animals that exhibit a preference for an environment previously paired with food, morphine, or cocaine show increased Fos expression in orexin neurons (Harris et al., 2005). Moreover, the amount of Fos expression in orexin neurons in the LLH is positively correlated with the amount of time animals spend in the drug paired chamber (Harris et al., 2005). The conditioning phase of a morphine place preference paradigm is also associated with increased stimulation of orexin neurons specifically in the LLH (Harris et al., 2007). Conversely, cocaine CPP has been associated with decreased orexin mRNA levels specifically in the LLH (Zhou et al., 2008). Interestingly, administration of SB-334867 reduces the expression of morphine place preference (Harris et al., 2005; Sharf et al., Submitted), but not of cocaine place preference (Sharf et al., Submitted). We have also recently found that OKO and WT mice prefer a morphine-paired environment equally (Sharf et al., Submitted) whereas it has been previously reported that OKO mice fail to develop such a preference (Narita et al., 2006). Further investigation is needed to clarify this discrepancy.
Previous reports suggest a role for orexin in the formation of a place preference to a morphine-paired environment. Bilateral excitotoxic lesions of the LH block the acquisition of morphine CPP. Unilateral lesions of the LH and intra-VTA administration of SB-334867 on the contralateral side also block the development of morphine CPP (Harris et al., 2007). Together, these data suggest that orexin’s actions in the VTA are essential for the development of morphine place preference. A role for the VTA in the expression of morphine place preference has also been reported as intra-VTA SB-334867 suppresses the expression of morphine place preference (Narita et al., 2006).
3. Conclusions and hypotheses
3.1 Orexin and reward processing
The self-administration data summarized thus far suggest a role for orexin in the rewarding properties of some, but not all, drugs of abuse. Brain stimulation reward (BSR) is another useful behavioral tool that has been used to assess sensitivity to reward. Here, animals will lever-press for the delivery of intracranial electrical stimulation and animals will robustly respond for stimulation of the LH (Olds and Milner, 1954). Interestingly, orexin A administration has been shown to elevate ICSS thresholds, suggesting a decrease in reward sensitivity (Boutrel et al., 2005). In contrast, SB-334867 abolishes nicotine-mediated reductions in BSR thresholds, suggesting that blockade of Ox1r results in decreased sensitivity to reward (Hollander et al., 2008). Based on these findings, it remains inconclusive whether orexin mediated alterations in drug self-administration are directly related to reward processing.
Further evidence for the role of orexin in reward processing comes from findings of CPP and cue-induced reinstatement data suggesting that orexin functioning contributes to drug seeking when the rewarding stimulus is no longer present due to conditioned reward of drug-associated stimuli. These data are suggestive of a reward component; however such interpretations can be misleading. CPP data suggests that processing of environmental cues and drug-associated contexts, and drug-seeking induced by the presence of such cues, is mediated, at least in part, by the orexin system. However, this hypothesis fails to explain data demonstrating that activation of the orexin system results in spontaneous reinstatement of an extinguished response.
3.2 Stress response
Based on evidence gathered using the CPP test, it becomes evident that orexin signaling, particularly in the VTA, is important for the processing of drug-associated stimuli. However, some evidence suggests that orexin’s involvement in reinstatement of extinguished drug seeking may be mediated by a stress component. Following orexin administration, corticosterone levels (Ida et al., 2000a; Jaszberenyi et al., 2000; Kuru et al., 2000) and plasma ACTH (Kuru et al., 2000) levels are increased. Furthermore, orexin mRNA levels and Fos expression in orexin neurons are increased following stressors, such as immobilization, foot-shock, and cold stress (Ida et al., 2000b; Zhu et al., 2002). It has been reported that icv orexin A induced reinstatement of an extinguished cocaine seeking response is abolished when orexin is co-administered with a CRF antagonist (Boutrel et al., 2005). In addition, foot-shock stress induced reinstatement of cocaine self-administration is abolished by SB-334867 administration (Boutrel et al., 2005). These data suggest that drug seeking is induced by activation of the stress pathway. Interestingly, intra-VTA CRF antagonists do not block orexin-A induced reinstatement of cocaine-seeking and foot-shock stress induced cocaine reinstatement is not blocked by intra-VTA SB-334867 (Wang et al., 2009), suggesting that, at least at the level of the VTA, orexin and CRF have independent actions and that blockade of stress induced reinstatement by SB-334867 is not VTA mediated.
The distinction between stress mediated and reward mediated effects of orexins have been previously suggested. Fos activation in animals that prefer a place previously paired with food, cocaine, or morphine is specific to the LLH (Harris et al., 2005) whereas Fos activation in animals undergoing a stressful morphine withdrawal is specific to the DMH and PFA (Sharf et al., 2008). As previously mentioned, orexin action in the VTA has been implicated in CPP reward processing (Harris et al., 2007), but in response to withdrawal, the VTA fails to be affected by orexin (Sharf et al., 2008). Thus, distinct orexin neuron populations and neural circuits may mediate orexin’s role in stress or reward.
3.3 Hypothesis
Although orexin has been discovered over a decade ago (De Lecea et al., 1998; Sakurai et al., 1998), the literature on its role in drug dependence and addiction is far from conclusive. Different behavioral models have been used and different drugs have been investigated; however, not all drugs have been tested with all behavioral models. Orexin’s contribution to drug-related behaviors appears to be drug specific and it is therefore imperative to directly compare orexin-drug specific interactions.
Together, the available data suggest a role for orexin functioning in drug self-administration, drug-associated cue processing and reward, and stress responses during drug-abstinence in drug-dependent animals (both somatic withdrawal and reinstatement of extinguished drug seeking). Although orexin is linked with all three, these effects might be mediated by specific anatomical substrates and pathways. As previously proposed by Harris and Aston-Jones (2006), distinct orexin neuronal subpopulations may serve disparate functions. Reward- and cue-processing appears to be mediated by LLH orexin neurons (Harris et al., 2005; Harris and Aston-Jones, 2006) acting in the VTA (Harris et al., 2007), whereas drug-related stress or aversive responses appear to be mediated by DMH and PFA orexin neurons acting directly or indirectly through brain regions including, but not limited to, the NAc (Sharf et al., 2008), PVN (Kuru et al., 2000; Sakamoto et al., 2004), and central nucleus of the amygdala (Kuru et al., 2000; Sakamoto et al., 2004). Although much evidence supports a role for orexin functioning in VTA in reward processing (Harris and Aston-Jones, 2006; Harris et al., 2007), renewal of extinguished cocaine seeking appears to be VTA independent (Hamlin et al., 2008), suggesting that other brain regions are likely to be involved, such as the basolateral amygdala and infralimibic prefrontal cortex (Hamlin et al., 2008).
Since orexins discovery, it has been noted that orexin projections are found throughout the brain (Mondal et al., 1999; Nambu et al., 1999) and that orexin receptors are present in multiple brain regions (Lu et al., 2000; Marcus et al., 2001; Trivedi et al., 1998). Interestingly, it has also been shown that the orexin receptors show somewhat different patterns of expression, where a brain region would express only Ox1r and another express only Ox2r (Marcus et al., 2001; Trivedi et al., 1998). The widespread orexin innervation and different expression of orexin receptors suggest that orexin plays many, perhaps unrelated, functions.
For instance, orexin has been shown to increase feeding in rodents (Ida et al., 1999; Lubkin and Stricker-Krongrad, 1998; Sakurai et al., 1998; Sweet et al., 1999) and other species (Dyer et al., 1999; Volkoff et al., 1999). The precise mechanism, be it metabolic, satiety, or reward, remains unclear. The role of orexin in food reward has also been reported. Central orexin A administration stimulates intake of a high fat diet more pronouncedly than intake of a low fat diet (Clegg et al., 2002) and treatment with SB-334867 decreases self-administration of high fat food pellets (Nair et al., 2008) and sweet pellets (Thorpe and Kotz, 2005).
Orexin has also been shown to mediate sleep and arousal processes. Canine narcolepsy has been attributed to a genetic disruption of the Ox2r gene (Lin et al., 1999) and OKO mice demonstrate behavioral arrests similar to those seen in narcoleptic episodes (Chemelli et al., 1999). Moreover, icv orexin results in increased locomotor activity (Hagan et al., 1999; Nakamura et al., 2000) and orexin has been implicated in vegetative biological functions such as regulation of body temperature and heart rate (Zheng et al., 2005)
Although the connectivity of orexin’s multiple functions remains debatable, different anatomical targets and orexin receptor expression suggest that different behaviors are mediated by distinct orexin neurons and circuits. However, as with all neurochemicals and pathways that mediate addiction, it may be that drugs of abuse act via a specific component of this neuropeptide system that otherwise serves to integrate behaviors, such as arousal, motivation and the formation of cue associations. More research is needed to elucidate specific neuroanatomical substrates and receptor mechanisms of orexin’s role in drug dependence and addiction. Moreover, more analysis of orexin’s role in non-drug rewards (e.g. food and sex) will help to more completely describe orexin’s influence on a diverse set of motivated and reward-related behaviors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56:112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–84. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–37. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 1999;143:39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, De Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-A, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–7. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett F.S.n., Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–6. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–9. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- Dyer CJ, Touchette KJ, Carroll JA, Allee GL, Matteri RL. Cloning of porcine prepro-orexin cDNA and effects of an intramuscular injection of synthetic procine orexin-B on feed intake in young pigs. Domest Anim Endocrinol. 1999;16:145–8. doi: 10.1016/s0739-7240(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–80. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–11. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural Brain Research. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–5. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–6. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Kuroiwa T, Fukui K, Nakazato M, Murakami T, Murakami N. Both corticotropin releasing factor and neuropeptide Y are involved in the effect of orexin (hypocretin) on the food intake in rats. Neurosci Lett. 2000a;293:119–22. doi: 10.1016/s0304-3940(00)01498-1. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000b;270:318–23. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Jaszberenyi M, Bujdoso E, Pataki I, Telegdy G. Effects of orexins on the hypothalamic-pituitary-adrenal system. J Neuroendocrinol. 2000;12:1174–8. doi: 10.1046/j.1365-2826.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–9. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–80. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiologica Scandinavia. 1974:412. [PubMed] [Google Scholar]
- Lu X-Y, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messanger RNA in the brain upon fasting. Horm Behav. 2000;37:335–44. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253:241–5. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martin G, Fabre V, Siggins GR, De Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104:111–7. doi: 10.1016/s0167-0115(01)00354-8. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Nakazato M, Date Y, Murakami N, Yanagisawa M, Matsukura S. Widespread distribution of orexin in rat brain and its regulation upon fasting. Biochem Biophys Res Commun. 1999;256:495–9. doi: 10.1006/bbrc.1999.0362. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Repeated amphetamine administration induces Fos in prefrontal cortical neurons that project to the lateral hypothalamus but not the nucleus accumbens or basolateral amygdala. Psychopharmacology. 2008;197:179–89. doi: 10.1007/s00213-007-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: An in vitro study. Peptides. 2009 doi: 10.1016/j.peptides.2009.04.018. In Press. [DOI] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. British Journal of Pharmacology. 2008;154:406–16. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–60. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake T, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–495. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Pasumarthi R, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–6. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77:367–73. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Priesty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;253:231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118:183–91. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–65. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.03.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin Mediates Morphine Place Preference, but not Morphine-Induced Hyperactivity or Sensitization. Submitted. [DOI] [PMC free article] [PubMed]
- Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–8. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–62. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–5. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiologica. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Geo XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neursci. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz N, Schmeichel B, Berridge C. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–40. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–95. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Volkoff H, Bjorklund JM, Peter RE. Stimulation of feeding behavior and food consumption in the goldfish, Carassius auratus, by orexin-A and orexin-B. Brain Res. 1999;846:204–9. doi: 10.1016/s0006-8993(99)02052-1. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of Cocaine Seeking by Hypocretin (Orexin) in the Ventral Tegmental Area: Independence from the Local Corticotropin-Releasing Factor Network. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chen X, Song C, Ye J, Yu Z, Hu Z. Postsynaptic excitation of prefrontal cortical pyramidal neurons by hypocretin-1/orexin A through the inhibition of potassium currents. J Neurosci Res. 2005;82:729–36. doi: 10.1002/jnr.20667. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103:400–7. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485:127–42. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–45. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–34. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13:1351–3. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]