Figure 2.
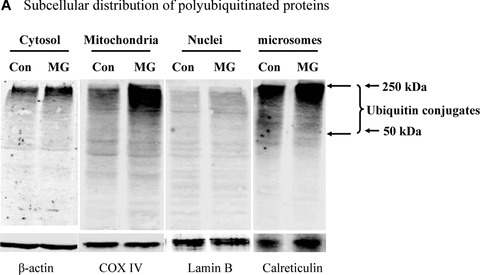
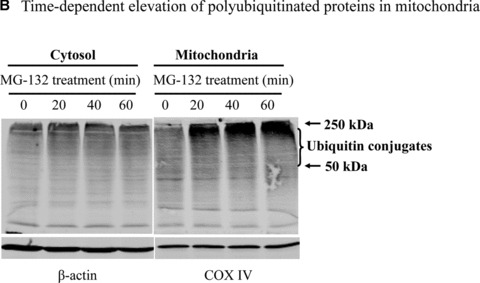
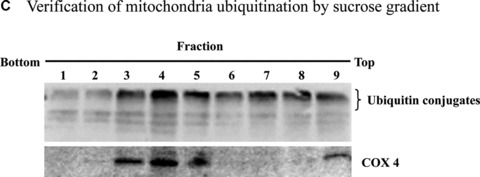
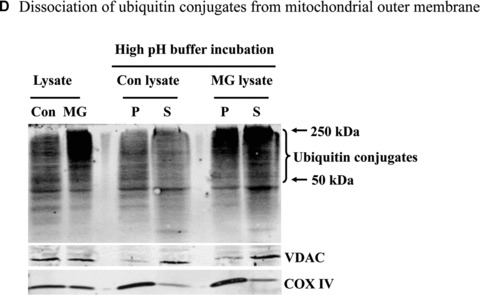
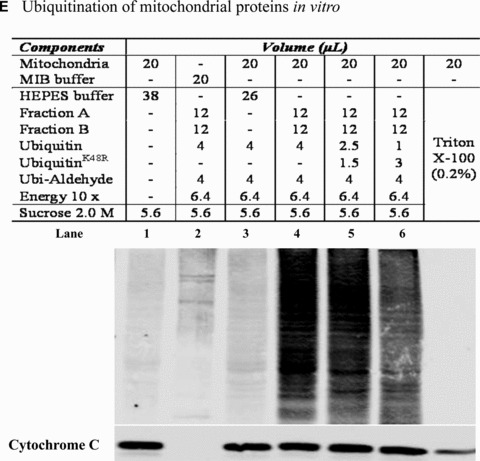
Mitochondrial accumulation of ubiquitinated proteins. (A) Subcellular distribution of polyubiquitin proteins. N27 cells were treated with 2.5 μM MG‐132 for 40 min. The cytosolic, mitochondrial, nuclear and microsomal fractions were prepared as described in the methods. Equal amounts of proteins were used for Western blot analysis using antibodies against ubiquitin, and subcellular markers β‐actin (cytosol), COX IV (mitochondria), lamin B (Nuclei) and calreticulin (microsome). (B) Time‐dependent elevation of polyubiquitinated proteins in mitochondria. Cytosolic and mitochondrial fractions were prepared from N27 cells exposed to 2.5 μM MG‐132 for 20, 40 or 60 min., and processed for ubiquitin immunoblotting. (C) Verification of mitochondria ubiquitination by sucrose gradient. Crude mitochondria isolated from N27 cells exposed to MG‐132 (2.5 μM for 40 min.) were subjected to sucrose gradient separation as described in the methods section. All the fractions collected were resolved on SDS‐PAGE and blotted with ubiquitin, β‐actin and/or COX IV antibodies. (D) Dissociation of ubiquitin conjugates from mitochondrial outer membrane. Mitochondria isolated from vehicle (Con) or MG‐132‐treated cells (MG) were incubated with a high pH buffer as described in the methods section, and then mitochondria were spun down. Both the mitochondrial pellets (P) and the equal proportion of the supernatant (S) were separated on SDS‐PAGE and immunoblotted with antibodies for ubiquitin, VDAC and COX IV. (E) Ubiquitination of mitochondrial proteins in vitro. The reaction was carried out by incubating the mitochondrial suspension (4 mg/ml) with ubiquitination enzymes (9.6 μg for fraction A and B included in the kits), ubiquitin (8 μg), ubiquitin aldehyde and an energy source. Mitochondria were then recovered from the reaction mixture for Western blotting using ubiquitin or cytochrome c antibodies.
