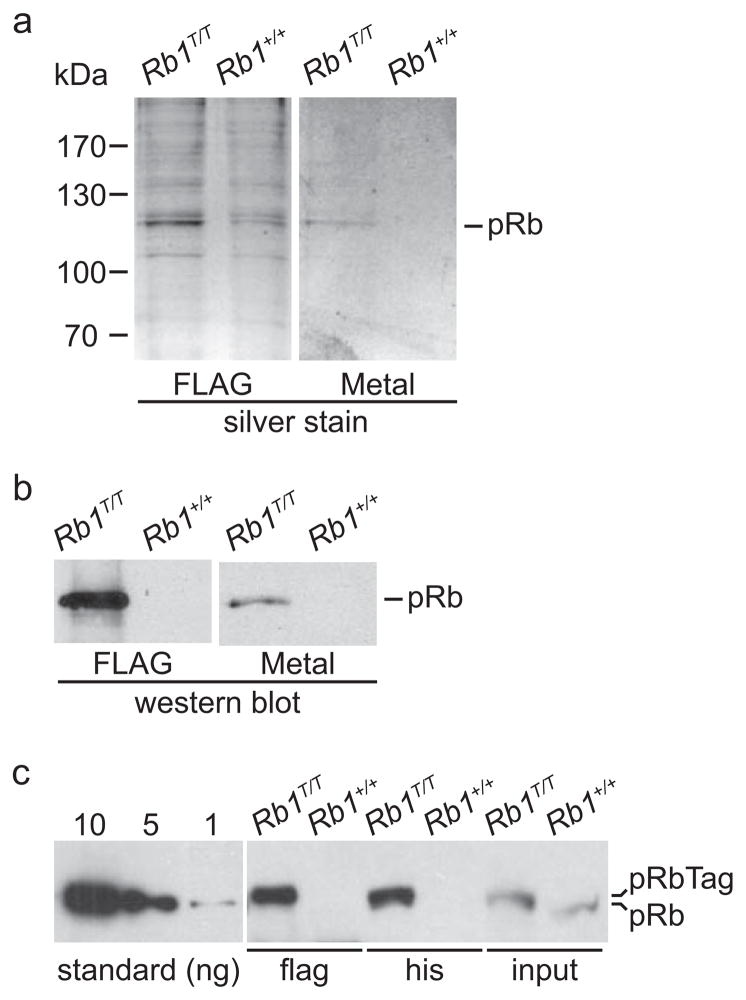
Figure 3. Purification of pRb from Rb1T/T mice.
A) Spleen nuclear extracts from mice of the indicated genotypes were purified using sequential FLAG immunoaffinity (FLAG) and metal affinity (Metal) resins. Material eluted from the indicated resin was analyzed by SDS-PAGE and the gels silver stained. The position of molecular weight standards is indicated. The position of the pRb band is also indicated. B) Aliquots of the purified extracts were analyzed for the presence of pRb by western blotting. C) Rb1 protein was purified from spleen nuclear extract from mice of the indicated genotypes by either FLAG immunoaffinity or histidine metal chelate chromatography and the material analyzed by western blotting. Input represents western analysis of 15% of the nuclear extract used for chromatography. The left panel shows western blot analysis of known quantities, in nanograms, of pRb purified from a bacterial expression system. Although separated, all samples were analyzed on the same western blot.