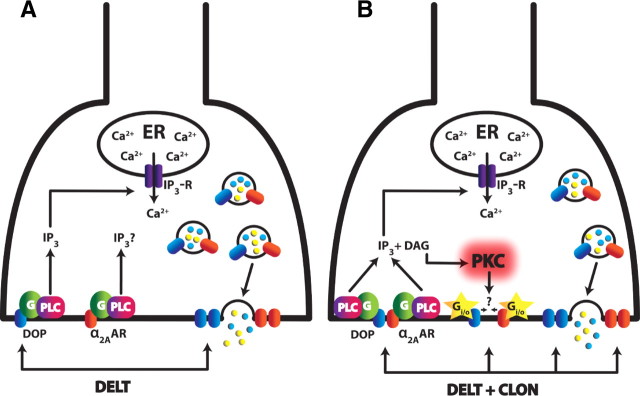
Figure 8.
Proposed mechanism of CLON-DELT analgesic synergy localized to primary afferent terminals in the dorsal horn of the spinal cord. A, Model of DOP agonist-induced DOP insertion coinciding with neuropeptide release (adapted from Bao et al., 2003). Activation of DOPs through administration of DELT causes activation of PLC (presumably through Gq), thereby increasing intracellular Ca2+ concentrations via IP3 receptors. This spike in Ca2+ mediates exocytosis of LDCVs, thus releasing neuropeptides and inserting intracellular “reserve” DOPs to the plasma membrane. B, Proposed model of CLON-DELT synergy mediated by PKC through coactivation of α2ARs and DOPs. Coactivation of α2ARs and DOPs causes neuropeptide release at a 100-fold lower concentration than DELT administration alone via the same mechanism as A. In contrast to DOP agonist-induced DOP insertion, however, coactivation of α2ARs and DOPs causes activation of PKC through increased levels of DAG. Activation of PKC, in turn, mediates the synergistic interaction of α2ARs and DOPs. One of several hypotheses for this mechanism is that the phosphorylation target(s) of PKC allow enhanced Gi/o coupling of both the α2AAR and DOP (yellow stars). An alternative hypothesis is that activation of PKC favors the formation of α2AR/DOP heterodimers with an enhanced inhibitory mode of action.