Figure 5.
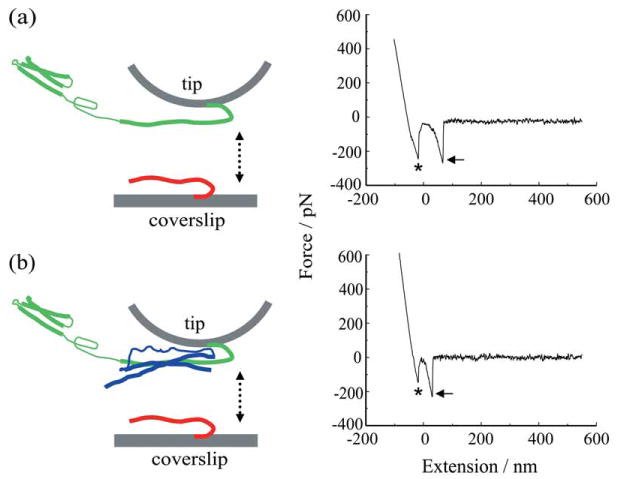
Force spectroscopy of SNARE proteins. (a) Recombinant synaptobrevin 2 (Sb2-His6; red) is attached to the nickel coated coverslip surface through histidine residue tags (His6) at its C terminus leaving its cytoplasmic domain free to interact with recombinant syntaxin 1A (Sx1A-His6; green) which is similarly attached by means of a C-terminus His6 tag to the nickel-coated cantilever tip. These two proteins are brought into close proximity (approach; arrow pointing down) by means of the piezoelectric element and then taken apart (retract, arrow pointing up). The retraction part of a typical force-distance (extension) curve using a Sx1A-His6 functionalized tip and a Sb2-H6 functionalized coverslip (right). Asterisk indicates the segment of the curve while the coverslip and the cantilever tip are still in contact. The arrow indicates the rupture of the Sx1A-Sb2 intermolecular »bond.« (b) SNAP25B (blue) reduces the extension of Sx1A-Sb2. Co-functionalized tips with equimolar ratio of Sx1A-His6/His6-SNAP25B were used to probe Sb2-His6 functionalized coverslips as shown in the force-distance curve (right). Asterisk as in (a); arrow, the rupture of the ternary complex intermolecular interaction. Retraction velocity, 1.6 μm/s. The drawings are not to scale. Modified from Ref. 16.
