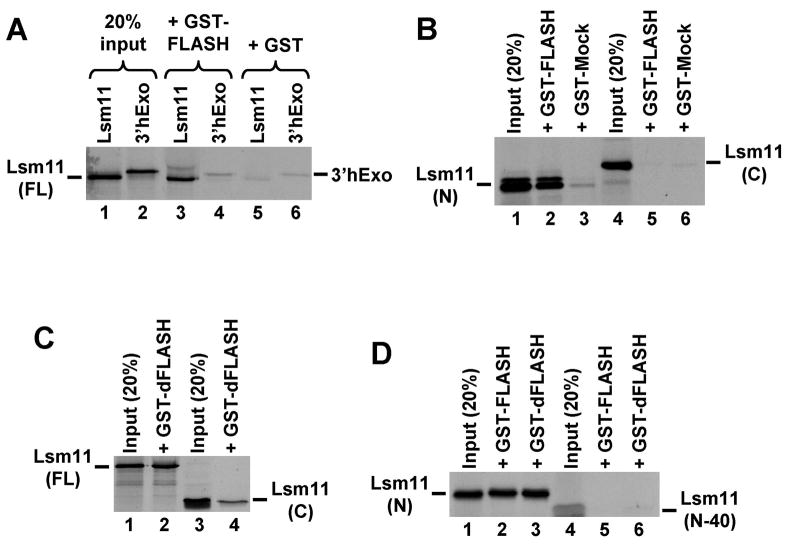
Figure 3. Human and Drosophila FLASH interact with human Lsm11 in vitro.
A. Full length human Lsm11 and 3′hExo were synthesized and labeled by in vitro translation with 35S-methionine and incubated with a fusion protein consisting of GST and the N-terminal portion of FLASH (lanes 3 and 4), or with GST alone (lanes 5 and 6). Lanes 1 and 2 contain 20% of each radioactive protein used in the pull-down assay. B. The ability of GST-FLASH and GST-Mock fusion proteins to interact with the 35S-labeled Lsm11 N-terminal half (lanes 2 and 3), or C-terminal half (lanes 5 and 6). Lanes 1 and 4 contain 20% of each radioactive protein used in the pull-down assay. C. The ability of GST fused to the N-terminal region of Drosophila FLASH (GST-dFLASH) to interact with the 35S-labeled full length (lane 2) or C-terminal half of human Lsm11 (lane 4). Lanes 1 and 3 contain 20% of each radioactive protein used in the pull-down assay. D. The ability of GST-FLASH and GST-dFLASH fusion proteins to interact with the 35S-labeled N-terminal half (lanes 2 and 3) or the N-terminal half lacking the first 40 amino acids of human Lsm11 (lanes 5 and 6). Lanes 1 and 4 contain 20% of each radioactive protein used in the pull-down assay.