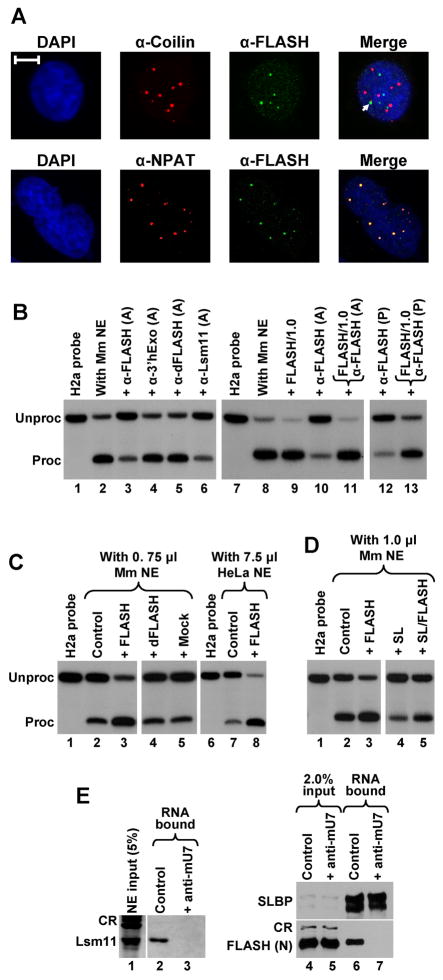
Figure 7. FLASH localizes to histone locus bodies and is required for 3′ end processing in mammalian nuclear extracts.
A. Immunofluorescence images of HeLa cells indirectly stained with anti-FLASH polyclonal antibody (green) and either the anti-coilin monoclonal antibody (red) or anti-NPAT monoclonal antibody (red). Nuclei were visualized with DAPI. The arrow indicates colocalization of Cajal and FLASH bodies. The bar represents 10 μm. B. The anti-FLASH antibody inhibits in vitro 3′ end processing of the mouse H2a histone pre-mRNA in a nuclear extract from mouse myeloma cells. Processing was carried out under control conditions (lanes 2 and 8) or in the presence of protein A-purified (A) or affinity-purified (P) antibodies, as indicated at the top of each lane. Processing reactions shown in lanes 11 and 13 contain both the antibody and 1 μg of the competing N-terminal FLASH (amino acids 1–139) fused to GST, whereas the processing reaction shown in lane 9 contains only 1 μg of the protein. Lanes 1 and 7 contain the input H2a substrate. C. The N-terminal fragment of human FLASH stimulates 3′ end processing of the H2a histone pre-mRNA in 0.75 μl of a mouse myeloma nuclear extract (lanes 2–5) and in 7.5 μl of a HeLa nuclear extract (lanes 7–8). Processing was carried out under control conditions (lanes 2 and 7), in the presence of 40 ng of the human N-terminal FLASH (lanes 3 and 8), or in the presence of 40 ng of control GST fusion proteins (lanes 4 and 5). D. Stimulation of 3′ end processing in 1 μl of a mouse nuclear extract by the N-terminal fragment of human FLASH (40 ng) in the absence (lane 3) or in the presence of a molar excess of the stem-loop RNA (lane 5). E. Processing complexes were assembled on a 5′ biotinylated histone pre-mRNA (0.25 μg) under normal conditions (lanes 2 and 6) or in the presence of the anti-mU7 oligonucleotide (lanes 3 and 7) and purified on streptavidin beads. The presence of individual proteins bound to histone pre-mRNA was detected by specific antibodies. Processing complexes analyzed in lanes 6 and 7 were assembled in a mouse nuclear extract supplemented with the N-terminal fragment of FLASH. CR indicate cross-reacting proteins recognized by an anti-Lsm11 antibody (lane 1) or anti-FLASH antibody (lane 4 and 5).