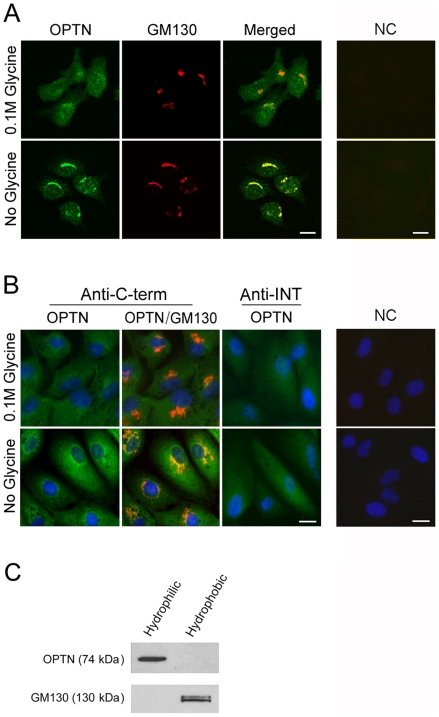
Figure 2. The endogenous optineurin is localized partially to the Golgi apparatus but is not a membrane protein.
A. RGC5 cells were double immunostained with anti-C-terminal optineurin (in green) and anti-GM130 (Golgi marker, in red). The endogenous optineurin (OPTN) had a diffuse cytoplasmic distribution pattern when 0.1 M glycine was included in the PBS rinsing buffer (top, left panel). Without glycine (bottom, left panel), the optineurin staining was prominent in the perinuclear region, colocalizing (in yellow, Merged) with the Golgi apparatus. Cells incubated with only secondary antibodies as negative controls (NC) showed no staining (right panels). B. Human RPE cells were immunostained with anti-C-terminal (Anti-C-term, in green) or anti-INT (Anti-INT, in green) optineurin along with anti-GM130 (in red). The endogenous optineurin (OPTN) had a diffuse cytoplasmic distribution pattern when anti-INT optineurin antibody was used (middle right panels) regardless whether glycine was included in the initial rinse. Using anti-C-terminal antibody, the optineurin staining was more perinuclar and Golgi-associated without the glycine rinse (left panels). Cells incubated with only secondary antibodies as negative controls (NC) showed minimal staining (right panels). The nuclei were stained with DAPI in blue. Scale bar, 10 µm. C. Western blot analyses of hydrophilic and hydrophobic fractions from RGC5 cell lysates. Total proteins in RGC lysates were subjected to membrane protein extraction. The hydrophilic fraction (left lane) contained predominantly non-membrane cytosolic proteins and the hydrophobic fraction (right lane) contained membrane proteins. Optineurin (OPTN) was detected exclusively in the hydrophilic fraction. GM130, a Golgi marker used as membrane protein positive control, was detected in the hydrophobic fraction as expected.