Figure 3.
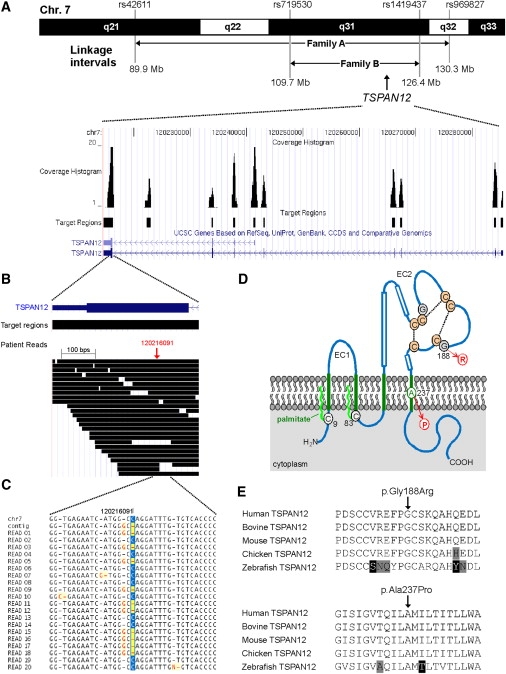
Genomic Structure, Next-Generation Sequencing, and Mutation Analysis of TSPAN12
(A) Upper panel, part of chromosome 7 showing the linkage intervals and the corresponding flanking SNPs for FEVR families A and B. The linkage interval from family A was enriched for targeted next-generation sequencing technology. The TSPAN12 gene is located in the shared interval between families A and B. Lower panel, overview of the genomic structure of the TSPAN12 gene, showing all introns and exons. For all exons, as well as a highly conserved region between exons 7 and 8, the sequence coverage histograms are presented.
(B) Overview of all patient reads for exon 8 of TSPAN12. In total, more than 30 reads were representing parts of this exon.
(C) Sequence traces for the twenty unique reads covering the TSPAN12 heterozygous G-to-C mutation. For these 20 reads, ten were representing the reference allele and ten were showing the mutant allele. At the protein level, the G-to-C transition results in the substitution of a proline for an alanine residue (c.709G>C [p.Ala237Pro]).
(D) Topology of TSPAN12 and the positions of the missense mutions identified in the FEVR families. TSPAN12 shows the characteristic domain structure of tetraspanins, including four transmembrane (TM) domains (in green). A cysteine residue preceding TM domain 1 (C9) and another following TM domain 2 (C83) are probably palmitoylated (bright green wavy lines). The second extracellular loop (EC2) contains three α helices (boxes), the typical C-C-G motif characteristic for all tetraspanin proteins, followed by one pair and two single cysteine residues. These six cysteines are predicted to form disulphide bonds (stippled lines). The glycine residue that is mutated into an arginine (G188) precedes one of these cysteines. The alanine residue that is mutated into a proline (A237) resides within an α-helical structure within the fourth TM region.
(E) Sequence comparison of a number of vertebrate TSPAN12 proteins. Depicted are parts of TSPAN12 that harbor the amino acid residues that are mutated. Both the glycine residue at position 188 (upper panel) and the alanine residue at position 237 (lower panel) are completely conserved throughout vertebrate evolution. Residues identical in all sequences are black on a white background, whereas similar amino acids are black on a light gray background. Nonconservative changes are indicated in white on a black background. UniProt accession number of the protein sequences used for sequence comparison are as follows: human TSPAN12, O95859; bovine TSPAN12, Q29RH7; mouse TSPAN12, Q8BKT6; chicken TSPAN12, Q5ZIF5; zebrafish TSPAN12, Q7T2G0.
