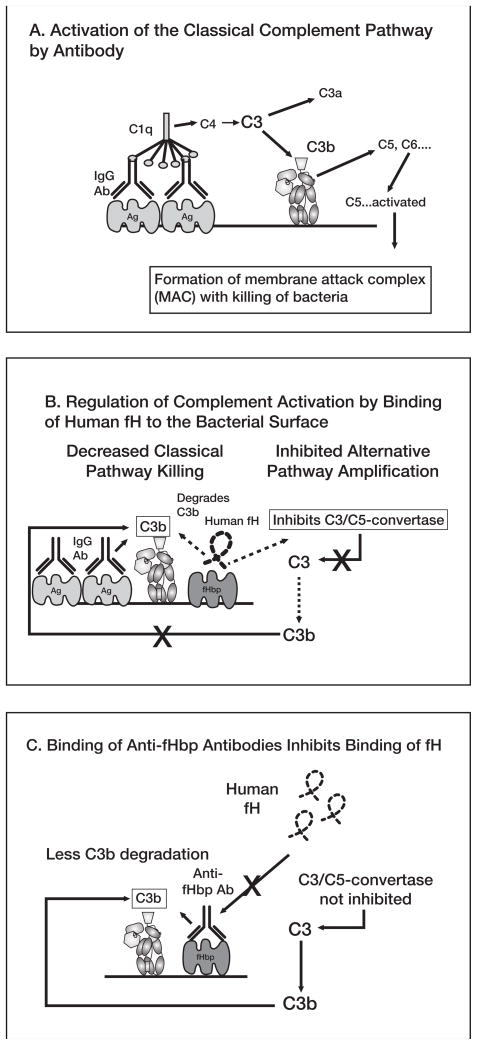
Figure 4.
A, Activation of classical complement pathway. Binding of 2 optimally spaced IgG molecules to the bacterial surface engages C1q and activates the classical complement pathway, which results in increased deposition of C3b. Bound C3b can serve as an opsonin and can also lead to bacteriolyis by cleavage of C5 and assembly of the C5b-9 membrane attack complex. Not shown are the components of the alternative pathway, which can be activated by the classical pathway and serve as an amplification loop. B, Regulation of complement activation by binding of human factor H (fH) to the bacterial surface. Human fH binds to surface-exposed fHbp. fH accelerates the decay of alternative pathway C3/C5 convertases, which downregulates the positive feedback amplification loop of the alternative pathway. Binding of fH also leads to degradation of C3b by factor I (not shown), which decreases classical pathway activation and amplification by the alternative pathway. C, Binding of antibodies to fHbp activates classical complement pathway bacteriolysis and also inhibits binding of fH to the bacterial surface. With decreased amounts of fH bound to the bacterial surface, there is less downregulation of complement activation and the organism becomes more susceptible to complement-mediated bacteriolysis.