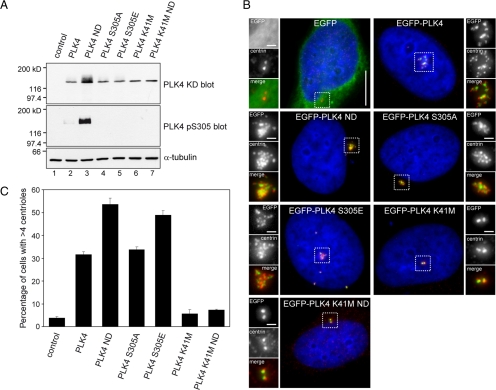
Figure 7.
Autophosphorylation enhances centriole amplification. (A) RPE1 cells were transiently transfected with pEGFP-PLK4 constructs and Western blotting was carried out using anti-PLK4 KD and anti-PLK4 pS305 antibodies to determine total and S305 phosphorylated PLK4 levels. An anti-α-tubulin blot was carried out as a loading control. The results show that mutation of S305, either to mimic or prevent phosphorylation, has no impact upon the stability of the kinase. Wild-type PLK4 ND containing two mutations in the degron motif, S285A and T289A, to prevent phosphorylation was expressed to high levels, whereas PLK4 K41M and PLK4 K41M ND were both expressed at a lower level, indicating that kinase activity may influence protein stability. Western blotting with the PLK4 pS305 antibody shows PLK4 ND is heavily S305 phosphorylated and suggests that stabilization allows active kinase to accumulate. (B) HeLa cells were transiently transfected with EGFP-PLK4 (green) expression constructs, fixed, and stained with DAPI (blue) to label DNA and anti-centrin antibody (red). Bar, 10 μm; inset, 1 μm. (C) Results of three independent experiments quantifying the number of transfected cells exhibiting centriole amplification (n = 200). The results showed that wild-type PLK4 and PLK4 S305A triggered centriole amplification to a similar extent, whereas PLK4 S305E triggered centriole amplification more frequently, indicating that mimicking S305 autophosphorylation enhances centriole amplification. In agreement with published results in Drosophila S2 cells, PLK4 ND expression resulted in a higher incidence of centriole amplification compared with wild-type PLK4.