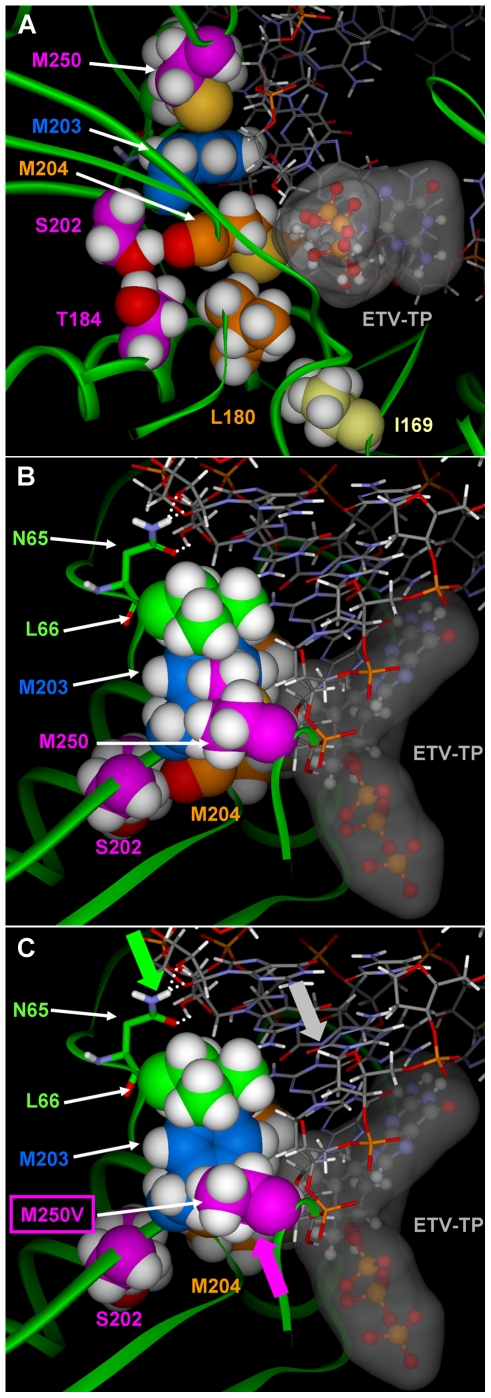
Figure 4. Position of residue M250 in the HBV RT/DNA molecular model.
A) The relative location of the M250, Y203, M204V, and other resistance residues are shown with primer-template DNA and ETV-TP in the dNTP binding site. The proximity of the M250 residue to both primer DNA and the Y203 residue are shown, indicating possible mechanisms for effects of M250 substitutions on the dNTP binding site, as well as the influence different side chains could have on primer positioning. B) M250 is packed against L66 and N65 forms hydrogen bonds (white dotted lines) to the hydroxyl groups on the backbone of the RNA template strand. C) The smaller side chain of the M250V no longer packs against L66. As indicated by the arrows (protein:green; DNA:gray; M250V:purple), the resulting conformational change in the protein to close the resultant hole repositions the RNA/DNA slightly over the NTP binding site. The slight modification to the NTP site enhances LVD binding while decreasing the binding affinity of ETV.