Figure 3.
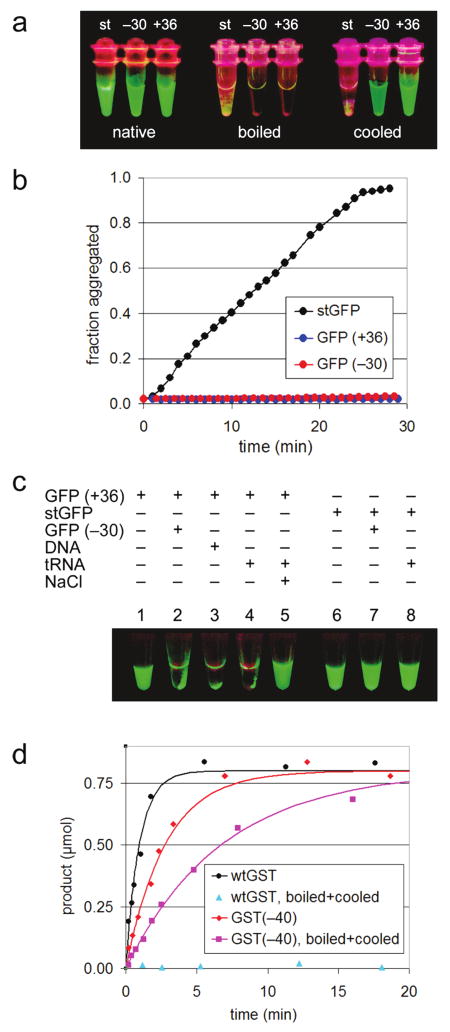
(a–c) Intermolecular properties of supercharged GFPs. (a) UV-illuminated samples of purified GFP variants (“native”), those samples heated 1 min at 100 °C (“boiled”), and those samples subsequently cooled for 2 h at 25 °C (“cooled”). (b) Aggregation of GFP variants was induced with 40% TFE at 25 °C and monitored by right-angle light scattering. (c) Supercharged GFPs adhere reversibly to oppositely charged macromolecules. Sample 1: 6 μg of GFP(+36) in 30 μL of 25 mM Tris pH 7.0 and 100 mM NaCl. Sample 2: 6 μg of GFP(−30) added to sample 1. Sample 3: 30 μg of salmon sperm DNA added to sample 1. Sample 4: 20 μg of E. coli tRNA added to sample 1. Sample 5: addition of NaCl to 1 M of sample 4. Samples 6–8: identical to samples 1, 2, and 4, respectively, except using stGFP instead of GFP(+36). All samples were spun briefly in a microcentrifuge and visualized under UV light. (d) Enzymatic activity of GST variants. Reactions contained 0.1 mg/mL of GST variant, 4 mM chloro-dinitrobenzene, 4 mM glutathione, and 100 mM potassium phosphate pH 6.5. Product formation was monitored at 340 nm, yielding reaction rates (vinit) of 1.0 μmol/min for wild-type GST, 0.36 μmol/min for GST(−40), and 0.15 μmol/min for GST(−40) after being boiled and cooled.
