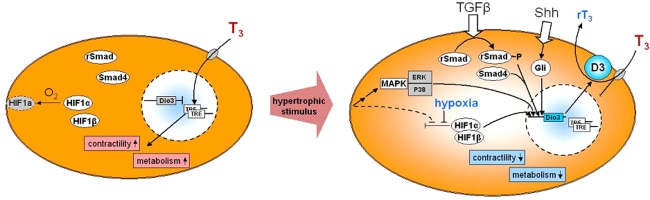
Fig. 2.
Schematic representation of some of the pathways that may contribute to the expression of D3 in pathologic ventricular remodeling. A normal cardiomyocyte is depicted on the left. T3 is taken up by specific transporters and genes that are transcriptionally regulated by T3 are characterized by the presence in their promoters of thyroid hormone response elements (TRE) to which the T3 receptor binds. HIF-1α is degraded under normoxic conditions, whereas HIF-1β is stable. Transition to the hypertrophic cardiomyocyte may be triggered by various stimuli (see text for details). Several signaling pathways converge on the mitogen activated protein kinases (MAPK), of which ERK and p38 activate DIO3 gene transcription. Mismatch of oxygen delivery and consumption, caused by ischemia and/or enlargement of the cardiomyocyte, results in hypoxia and stabilization of HIF-1α. Dimerization with HIF-1β forms the HIF-1 complex. HIF-1α may also be stabilized directly as a result of hemodynamic overload and mechanical stress. TGFβ stimulates the Smad signaling pathway by phosphorylation of R-Smad2 and -3, which form a complex with Smad4. Together with HIF-1, phosphorylated ERK, and p38 this results in the synergistic stimulation of transcription of the DIO3 gene. D3 expression is further stimulated by the secreted morphogen Sonic hedgehog (Shh) which signals through the Gli family of transcription factors. D3 activity converts T3 to the inactive metabolite reverse T3, resulting in reduced T3-dependent gene expression and a concomitant reduction of contractile activity and energy turnover