Figure 7. HYPE adenylylates RhoA, Rac, and Cdc42 on a Tyr residue.
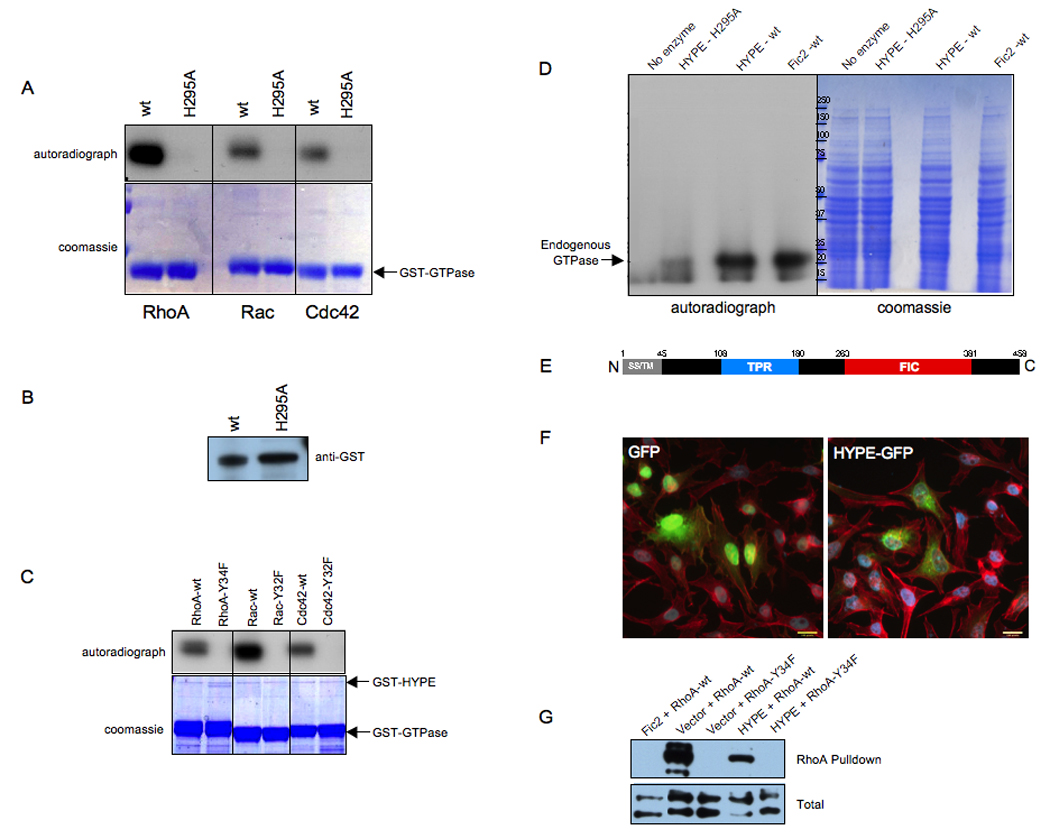
(A) HYPE adenylylates RhoA, Rac, and Cdc42. Bacterially expressed GSTHYPE or GST-HYPE H295A was incubated with wild type RhoA, Rac, and Cdc42 expressed as GST fusion proteins in bacteria in the in vitro adenylylation assay. Samples were separated on SDS-PAGE and visualized by autoradiography (top panel) and Coomassie staining (bottom panel). The GTPases are indicated by an arrow. Wild type HYPE adenylylates RhoA, Rac, and Cdc42 while the H295A mutant is catalytically inactive.
(B) GST-HYPE as visualized by western analyses. The reaction products were separated on SDS-PAGE, transferred into nitrocellulose membrane, and blotted with GST antibody.
(C) HYPE does not modify the switch I Tyr mutants of RhoA, Rac, and Cdc42. Bacterially expressed GST-HYPE was incubated with RhoA-Y34F, Rac-Y32F, and Cdc42-Y32F expressed as GST fusion proteins in bacteria in the in vitro adenylylation assay. Samples were separated on SDS-PAGE and visualized by autoradiography (top panel) and Coomassie staining (bottom panel). The positions that GST-HYPE and the GTPases run on the gel are indicated by arrows.
(D) HYPE modifies endogenous GTPases in HEK293T cell extracts. Membrane free HEK293T cell extracts were incubated with bacterially expressed GST-HYPE, GST-HYPE H295A, GST-Fic2 or without any enzyme. Samples were separated on SDS-PAGE and visualized by autoradiography (left panel) and Coomassie staining (middle panel). Like Fic2, HYPE activity is also directed against Rho GTPases in cellular extracts.
(E) Schematic representation of HYPE. The 458 amino acid protein contains a hydrophobic N-terminus (grey) consisting of a putative secretion signal (SS) and transmembrane (TM) domain, followed by a tetratricopeptide (TPR) repeat (blue) possibly involved in protein binding, and a Fic (FIC) domain (red).
(F) HYPE is not cytotoxic. Immunofluorescence microscopy was performed on HeLa cells transfected with the EGFP vector control and HYPE-GFP. Cell transfection and HYPE expression of the indicated constructs were visualized by EGFP fluorescence. Cell morphology was determined by staining actin filaments with rhodamine phalloidin. HYPE expression is not cytotoxic and does not induce a cell rounding phenotype.
(G) HYPE expression reduces binding of RhoA to its downstream effector, rhotekin. HeLa cells expressing HYPE-GFP or EGFP-Fic2 in conjunction with wild type FLAG-tagged RhoA or the FLAG-RhoAY34F mutant were lysed and used for affinity precipitation with 30 µg Rht-PBD (Experimental Procedures). The proteins bound to the beads as well as total extract samples were separated on SDS-PAGE, transferred to nitrocellulose membrane, and blotted with FLAG antibody.
