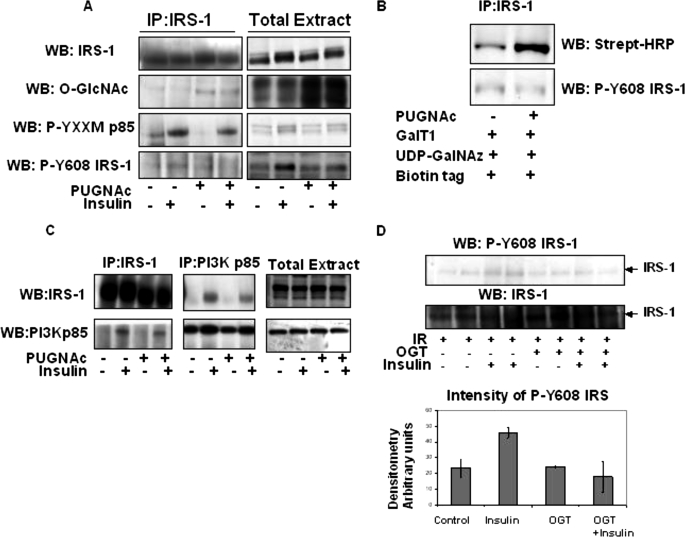
FIGURE 3.
IRS-1 is modified with O-GlcNAc, and elevations in O-GlcNAc reduce IRS-1 interaction with PI3K p85 and inhibit phosphorylation of the Tyr(P)608 IRS-1 YXXM motif. A, IRS-1 was immunoprecipitated (IP) from 3T3-L1 adipocytes pretreated or not with PUGNAc and then stimulated or not with 5 nm insulin and probed for IRS-1, O-GlcNAc RL-2, phospho-YXXM p85, and Tyr(P)612 IRS-1. The total cell extract was also Western blotted (WB) for all antibodies as a control. B, IRS-1 was immunoprecipitated from 3T3- L1 adipocytes pretreated or not with PUGNAc and labeled with GalT1, UDP-N-azidogalactosamine (GalNAz), and biotin tag and subjected to SDS-PAGE and Western blotting with streptavidin-horseradish peroxidase. C, IRS-1 and PI3K p85 were immunoprecipitated from PUGNAc-treated or -untreated and 5 nm insulin-stimulated or -unstimulated 3T3-L1 adipocytes and Western blotted for PI3K p85 and IRS-1 interaction, respectively. Total extracts were also Western blotted with IRS-1 and PI3K p85 as controls. Data are representative of at least three experiments IR, insulin receptor. D, immunopurified IRS-1 was labeled in vitro with UDP-[3H]GlcNAc by incubating with or without OGT, Sepharose A/G and washed, and then insulin receptor, stimulated or not with 1 μm insulin, was added for 20 min, and insulin-stimulated insulin receptor phosphorylation of Tyr(P)608 IRS-1 was measured by Western blot analysis using Tyr(P)608 IRS-1 antibody. Western blot intensity was quantitated using ImageJ.