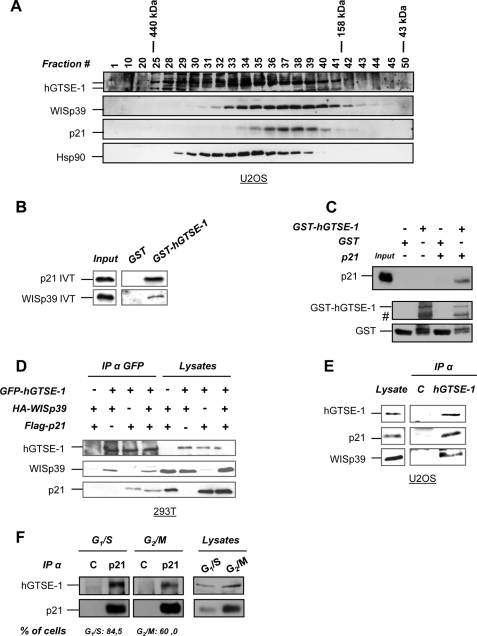
FIGURE 3.
Interaction of hGTSE-1 with p21 and the co-chaperone WISp39. A, U2OS extracts were resolved by gel filtration on a Superose 6 column, and the fractions were analyzed by immunoblotting. B, in vitro binding assay using recombinant GST or GST-hGTSE-1 fusion protein incubated with 35S-labeled IVT p21 or WISp39. The left panel (input) shows 20% of the input of IVT. C, in vitro binding assay using recombinant resin-bound GST or GST-hGTSE-1 incubated with recombinant p21 after thrombin-mediated removal of the GST tag. #, degradation bands of hGTSE-1. The left panel (input) shows 10% of p21 input. D, 293T cells were transfected with FLAG-p21, HA-WISp39, and GFP-hGTSE-1 for 24 h, followed by immunoprecipitation (IP) using anti-GFP antibody. E, immunoprecipitation of endogenous hGTSE-1 from U2OS cells carried out in ”low stringency“ lysis buffer with anti-hGTSE-1 or -GFP (C) antibody as control. F, U2OS cells were synchronized at G1/S or G2/M phases of the cell cycle and subjected to immunoprecipitation with anti-p21 or -HA (C) as control. Bottom, FACS quantification of the percentage of cells in the specific phases of the cell cycle. Immunoblot analyses of A, C, D, E, and F, were performed using antibodies against hGTSE-1, p21, WISp39, Hsp90, GST, FLAG, HA, and GFP.