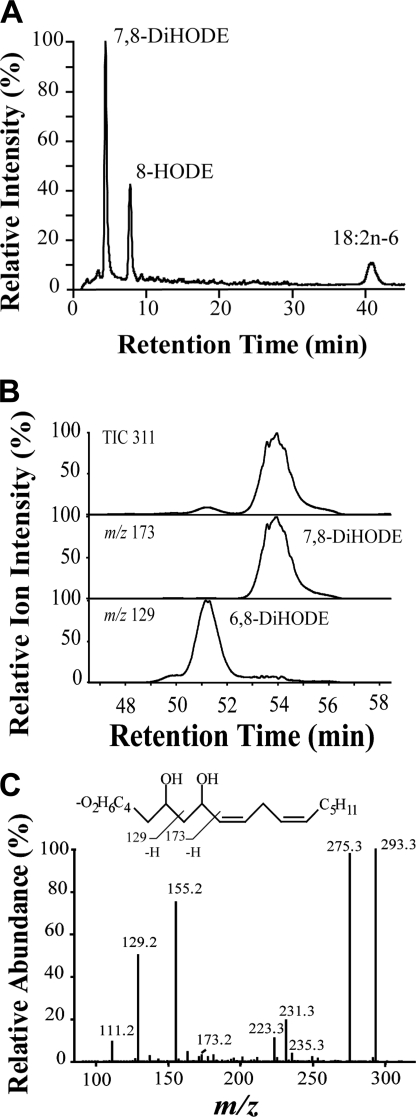
FIGURE 3.
RP-HPLC separation with MS/MS analysis of products formed from 18:2n-6 by M. oryzae Guy11. A, nitrogen powder of M. oryzae was incubated with (8S)-[2H]18:2n-6, and the major metabolites and unchanged substrate were analyzed by LC-MS (total ion current). The deuterium content of the metabolites and recovered 18:2n-6 was determined by MS/MS analysis (see Table 2). Small amounts of 6,8-DiHODE eluted on the left shoulder of 7,8-DiHODE. B, mycelia were incubated with 18:2n-6, and 6,8- and 7,8-DiHODE were separated by preparative RP-HPLC and analyzed by MS/MS. Top, total ion current (m/z 311→full scan); middle, reconstructed ion chromatogram for a characteristic ion of 7,8-DiHODE (m/z 173; −OOC-(CH2)5-CHOH-CHO), and bottom, a characteristic ion of 6,8-DiHODE (m/z 127; −OOC-(CH2)4-CHO). C, MS/MS spectrum of 6,8-DiHODE. Inset shows formation of characteristic fragments.