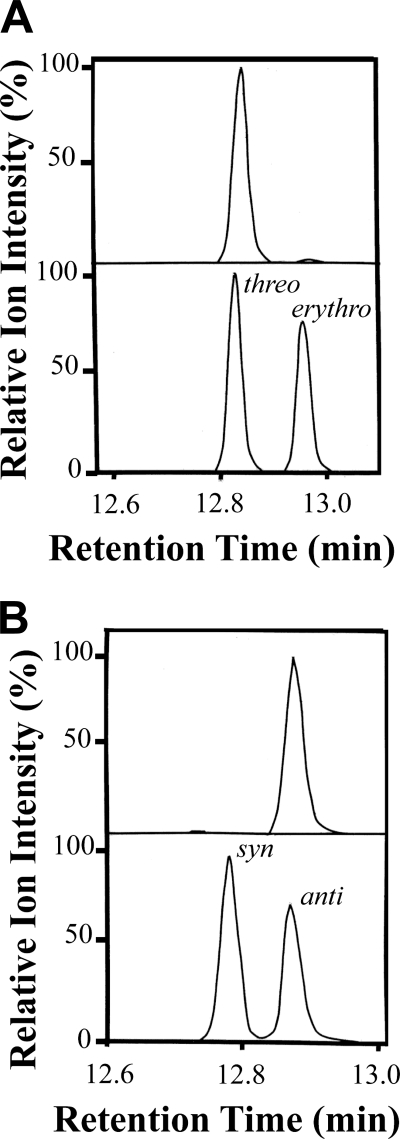
FIGURE 4.
Steric analysis of (7,8S)-DiHODE and (6,8R)-DiHODE by capillary GC-MS. A, top, chromatogram of (7,8S)-DiHODE methyl ester after hydrogenation and silylation with selective ion monitoring of m/z 231. Bottom, chromatogram of the methyl esters and TMS ether derivatives of erythro and threo 7,8-hydroxyoctadecanoic acid. As the precursor 8-HPODE has an R configuration, the results show that (7S,8S)-DiHODE was formed. B, top, chromatogram shows selective ion chromatogram of 6,8-DiHODE methyl ester after hydrogenation and silylation with selective ion monitoring of m/z 217. Bottom, selective ion chromatogram of the syn and anti stereoisomers of methyl 6,8-hydroxyoctadecanoate (TMS ether derivative). As the metabolite was formed from (8R)-HPODE, the results show that (6S,8R)-DiHODE was produced. The ion intensities were normalized to 100%, as indicated.