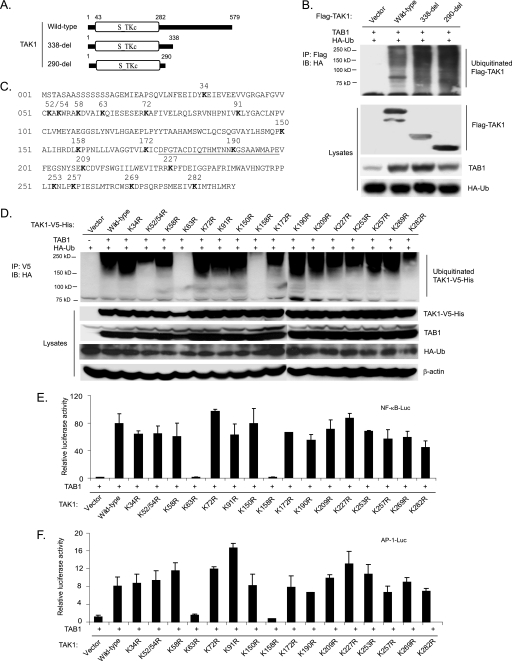
FIGURE 3.
Co-overexpression of TAK1/TAB1 induces Lys63-linked TAK1 polyubiquitination at the Lys158 residue within the kinase domain. A, schematic representation of TAK1 wild type and deletion mutants with the kinase domain indicated. B, co-overexpression of the TAK1/TAB1-induced TAK1 polyubiquitination site is located within the kinase domain. Expression vectors encoding HA-ubiquitin and TAB1 were co-transfected into HEK-293T cells with control vector and expression vectors encoding FLAG-TAK1 wild type and two deletion mutants (338-del and 290-del), respectively. FLAG-TAK1 proteins in the transfected cells were immunoprecipitated (IP) with anti-FLAG antibodies and immunoblotted (IB) with anti-HA antibodies to detect the presence of ubiquitinated FLAG-TAK1. C, TAK1 primary sequence with the lysine residues within its N-terminal 290 amino acids indicated. The kinase activation loop is underlined. D, co-overexpression of TAK1/TAB1 induces TAK1 polyubiquitination at Lys158 within the kinase domain. Expression vectors encoding HA-ubiquitin and TAB1 were co-transfected into HEK-293T cells with control vector and expression vectors encoding TAK1-V5-His wild type and 16 lysine to arginine mutants, respectively. TAK1-V5-His proteins in the transfected cells were immunoprecipitated with anti-V5 antibodies and immunoblotted with anti-HA antibodies to detect the presence of ubiquitinated TAK1-V5-His. E and F, the effect of overexpression of TAK1 lysine to arginine mutants with TAB1 on TAK1/TAB1-induced NF-κB (E) and AP-1 (F) activation. TAB1 expression vectors, NF-κB luciferase reporter, and control Renilla luciferase reporter vectors were co-transfected into TAK1-deficient MEF cells with empty vector or expression vectors encoding TAK1 wild type and lysine to arginine mutants, respectively. The relative luciferase activity was measured 48 h later and normalized with the Renilla activity. Error bars, ±S.D. in triplicate experiments.