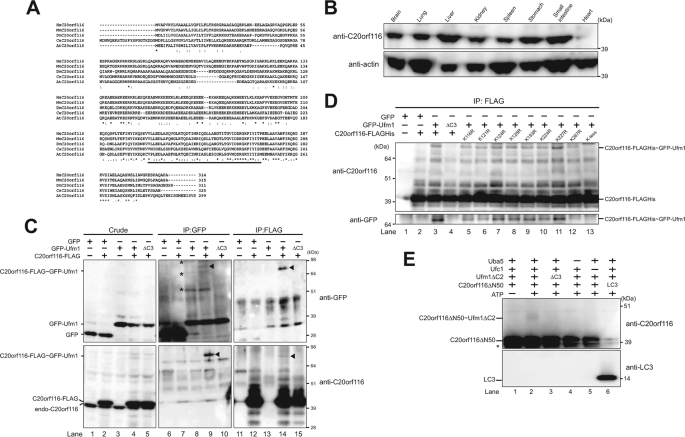
FIGURE 1.
C20orf116, a substrate for the Ufm1 modification system. A, sequence alignment of human C20orf116 (HsC20orf116) and its homologues of other species. The amino acid sequence of human C20orf116 was compared with those of other species by using the ClustalW program. *, indicate identical amino acids. Single and double dots indicate weakly and strongly similar amino acids, respectively. The underline indicates the PCI domain. B, tissue distribution of C20orf116. Homogenates prepared from various mouse tissues were analyzed by immunoblot with anti-C20orf116 and anti-actin antibodies. 20 μg of proteins were applied on each lane. C, C20orf116 is conjugated with Ufm1 in vivo. HEK293 cells were transfected with vectors at the indicated combinations. The cell lysates were immunoprecipitated (IP) with anti-GFP or anti-FLAG antibody, followed by immunoblot analysis with anti-GFP and anti-C20orf116 antibodies. The bands corresponding to GFP, GFP-Ufm1, endogenous C20orf116, C20orf116-FLAG, and C20orf116-FLAG·GFP-Ufm1 conjugate are indicated on the left. The arrowheads and asterisks indicate C20orf116-FLAG·GFP-Ufm1 conjugate and unknown proteins conjugated with GFP-Ufm1, respectively. D, determination of a lysine residue of C20orf116 for Ufm1 conjugation. GFP-Ufm1 was co-expressed with wild-type C20orf116 or the indicated mutants. The cell lysates were immunoprecipitated by anti-FLAG antibody and analyzed as described in C. E, in vitro reconstitution of C20orf116·Ufm1 conjugate. Purified recombinant C20orf116ΔN50 was incubated at 30 °C for 90 min with components of various combinations as indicated. The mixtures were boiled for 5 min with SDS-sample buffer containing 5% β-mercaptoethanol to stop the reaction. The samples were analyzed by immunoblot with anti-C20orf116 antibody. The bands corresponding to C20orf116ΔN50, LC3, and C20orf116ΔN50·Ufm1ΔC2 conjugate are indicated. *, degradative product of C20orf116ΔN50. The data shown in B–E are representative of three experiments with similar results.