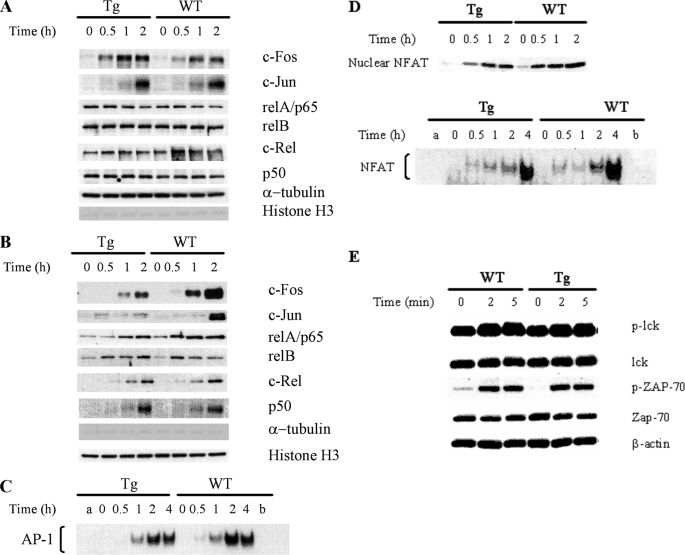
FIGURE 7.
AP-1 and NF-κB protein nuclear import in T cells according to immunoblotting and EMSA. WT and Tg spleen T cells were stimulated with PMA (5 nm) and ionomycin PMA (1 μg/ml) at 37 °C for various time periods as indicated. Cytosolic and nuclear proteins were then extracted from the cells. The nuclear and cytosolic fractions were resolved in 10% SDS gels and analyzed by immunoblotting using Abs against AP-1 and NF-κB members. α-Tubulin and histone H3 levels were used to monitor the cross-contamination of cytosolic proteins in the nuclear fraction and vice versa. The nuclear fractions were also employed in AP-1 EMSA. The experiments were performed at least twice, and representative results are shown. A, protein levels of AP-1 and NF-κB members in the cytosol of Tg and WT T cells remain constant during PMA and ionomycin stimulation according to immunoblotting. B, compromised AP-1 but not NF-κB members nuclear import in Tg T cells upon PMA and ionomycin stimulation according to immunoblotting. C, compromised AP-1 nuclear import in Tg T cells upon PMA and ionomycin stimulation according to EMSA. Nuclear protein extracted from PMA plus ionomycin-stimulated WT and Tg T cells were employed in AP-1 EMSA. Lane a is a control with no nuclear protein added. Competition was performed by incubating extracts with a 100-fold molar excess of unlabeled oligonucleotide before the addition of labeled probe (lane b). Data are representative of at least three independent experiments. D, moderately compromised NFAT nuclear import in Tg T cells. Nuclear protein extracted from PMA plus ionomycin-stimulated WT and Tg T cells were employed in NFAT EMSA. Lane a is a control with no nuclear protein added. Competition was performed by incubating extracts with a 100-fold molar excess of unlabeled oligonucleotide before the addition of labeled probe (lane b). Data are representative of at least three independent experiments. E, normal early TCR signaling in Ran Tg T cells. Ran Tg CD4 T cells were cross-linked by anti-CD3 and anti-CD4 for 0–5 min, and their Lck and ZAP-70 phosphorylation (indicated by p-Lck and p-ZAP-70) in total cell lysates was assessed by immunoblotting. Total Lck, ZAP-70, and β-actin were used to ascertain even protein loading.