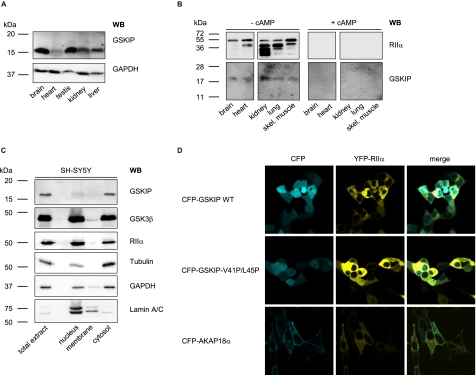
FIGURE 4.
GSKIP is widely expressed and functions as an AKAP in vivo. A, lysates from the indicated rat organs were subjected to Western blot (WB) analysis with an anti-GSKIP antibody and, as a loading control, anti-GAPDH antibody (167 μg of total protein per lane). B, cAMP-agarose pulldowns were obtained from the indicated rat tissue lysates (3 mg of total protein in each sample) in the absence (− cAMP) or presence (+ cAMP; 50 μm) of cAMP. RIIα subunits of PKA and GSKIP were detected with specific antibodies by Western blotting. skel., skeletal. C, indicated subcellular fractions were obtained from SH-SY5Y cells. 10 μg of total protein from each fraction was analyzed for the presence of the indicated proteins by Western blotting. D, GSKIP and RIIα co-localize in HEK293 cells. HEK293 cells were transiently transfected to express YFP-RIIα and fusions of CFP with wild type GSKIP (CFP-GSKIP WT), GSKIP-V41P/L45P, or as a plasma membrane marker AKAP18α.