FIGURE 1.
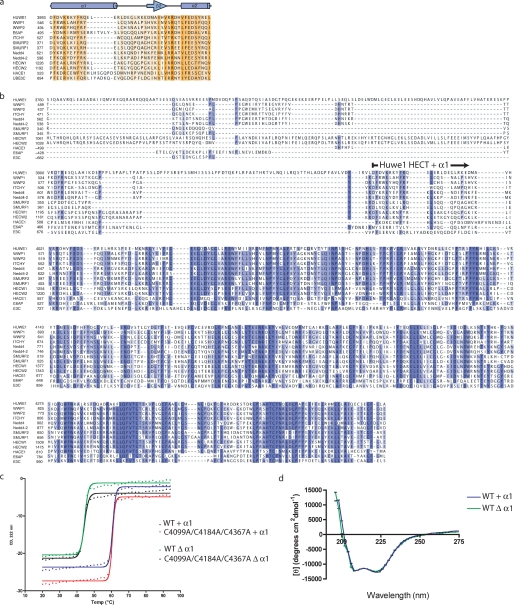
The α1 helix stabilizes the HUWE1 HECT domain. a, multiple sequence alignment of helix α1 with a diverse set of human HECT E3 ligases is shown. Residue conservation is indicated by degree of shading ranging from orange (most conserved) to light yellow (least conserved). Secondary structure is illustrated with α-helices as cylinders and β-sheets as arrows. b, shown is multiple sequence alignment with a diverse set of human HECT E3 ligases indicating that sequence conservation drops off N-terminal to the α1 helix. The N terminus of the HECT domain + α1 helix is indicated. c, thermostability of the HUWE1 HECT domain was measured in a CD melting experiment. HUWE1 HECT domain, +/Δ α1 helix, WT, or with cysteine mutations, was heated in a circular dichroism cuvette, and unfolding was measured at 222 nm as a loss of helical content. Deletion of helix α1 results in a drastic reduction of thermostability. d, a CD scan experiment demonstrates the structural similarities of the +/Δ α1 helix versions of the HECT domain.