FIGURE 2.
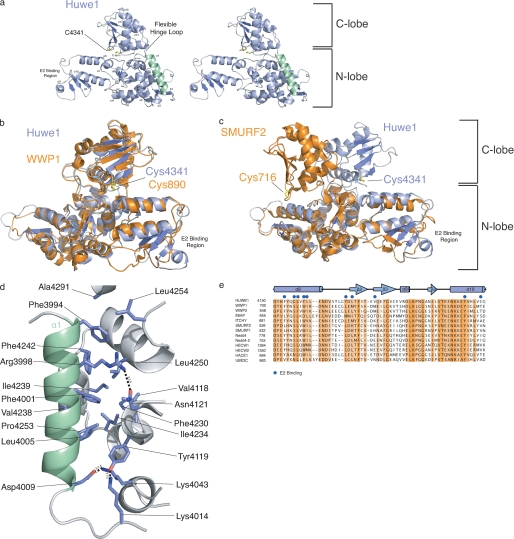
Structure of the HUWE1 HECT domain. a, a stereo view of HUWE1 HECT domain (residues 3993–4374) shows the N and C lobes connected by the hinge loop. Helix α1 is colored green. The N-lobe contains the E2 binding region, and the C-lobe contains the catalytic cysteine (Cys-4341). b, overlay of HUWE1 (blue) and WWP1 (orange; PDB 1ND7) crystal structures is shown. c, overlay of HUWE1 (blue) and Smurf2 (orange; PDB 1ZVD) crystal structures is shown. d, helix α1 plays a significant role in mediating hydrophobic contacts that maintain the core of the HUWE1 HECT domain. Hydrophobic residues in the α1 helix, Phe-3994 and Phe-4001, pack into hydrophobic pockets in the N lobe. Arg-3998 and Asp-4009 form hydrogen bonds stabilizing the N-lobe. Lys-4014 and Tyr-4119, C-terminal to the α1 helix, orient the α1 helix to further stabilize the N-lobe. e, multiple sequence alignment of E2 binding region with a diverse set of human HECT E3 ligases is shown. Residues important for E2 binding are indicated with blue circles.