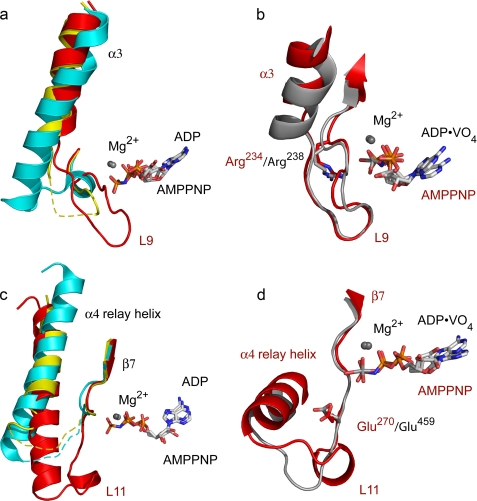
FIGURE 2.
Closed nucleotide-binding site. Comparisons of the α3 helix and switch I loop (a and b) and of the relay helix and switch II loops (c and d) from representative kinesin and myosin structures are shown. a, differences in α3 and L9 between Eg5·AMPPNP (red) with the equivalent sequences of Eg5·ADP (cyan) and Kif1A·AMPPNP (yellow). Disordered residues are shown as dashed lines. Note that L9 of Eg5·AMPPNP approaches the nucleotide in this closed conformation. b, Eg5·AMPPNP (red) and myosin·ADP·vanadate (gray) have similar switch I loop conformations. Conserved switch I arginines are shown. c, relay helices and switch II loops of Eg5·AMPPNP (red), Eg5·ADP (cyan), and Kif1A·AMPPNP (yellow). L11 of Eg5·AMPPNP is ordered, and the relay helix is in an ATP-like position. d, Eg5·AMPPNP (red) and myosin·ADP·vanadate (gray) have similar switch II loop conformations. Conserved switch II glutamates are shown.