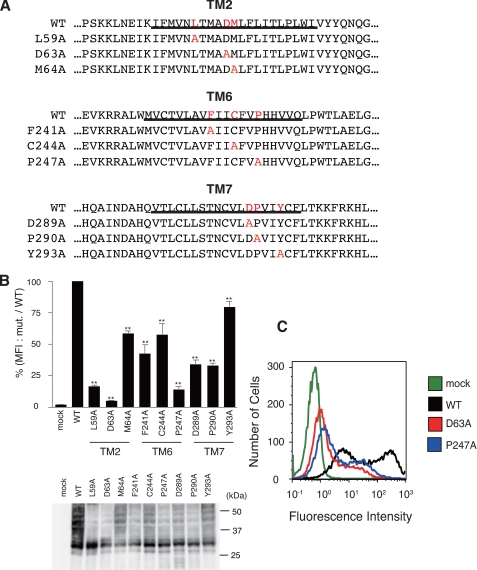
FIGURE 1.
Requirement of conserved residues in rhodopsin-type GPCRs for cell surface expression of HA-hPAFR. A, the 9 mutant HA-hPAFRs generated in this study are indicated. Conserved residues and substituted alanines in each mutant PAFR are indicated in red. Transmembrane domains are underlined. B, after transfection of WT and mutant HA-hPAFRs into HeLa cells, cell surface expression levels of each mutant HA-hPAFR were determined with flow cytometry. After the cells were stained with an anti-HA primary antibody and phycoerythrin-conjugated anti-rat IgG secondary antibody, the fluorescence intensity of each transfectant was measured. The mean fluorescence intensities (MFI), represented as a percentage of the MFI of WT HA-hPAFR, were used to evaluate the expression levels. Data are represented as mean ± S.E. (n = 3). Statistical significance was analyzed using analysis of variance with Dunnett post hoc pairwise comparisons. **, p < 0.01 (versus WT). Bottom, the protein levels of the tested receptors in HeLa cells were shown by Western blotting using anti-HA antibody. Approximate molecular sizes are examined in kDa at the right. C, a representative result from the flow cytometric analysis is shown. HeLa cells were transiently transfected with WT HA-hPAFR, D63A, P247A, or empty vector.