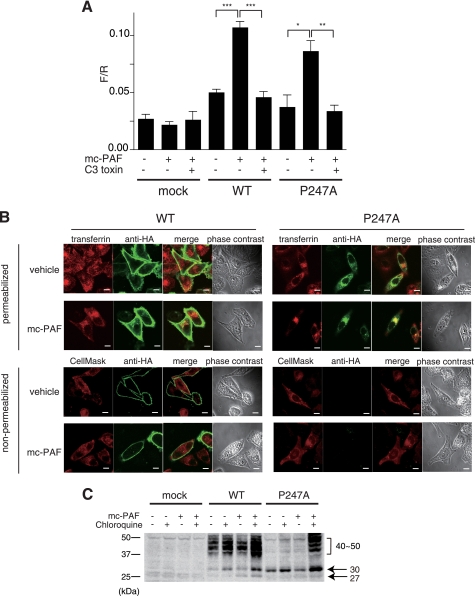
FIGURE 6.
Signaling and internalization of P247A after treatment with mc-PAF. A, PC12h cells were transiently transfected with reporter plasmids (zif 268-firefly luciferase and cytomegalovirus-Renilla luciferase as an internal control) and an HA-hPAFR expression plasmid with or without C3 exoenzyme (pEF-C3). The cells were cultured for 36 h after transfection and subsequently stimulated with 1 μm mc-PAF for 12 h. The ratios of firefly luciferase activity to Renilla luciferase are shown. Data are mean ± S.E. (n = 3). The data shown are representative of three independent experiments with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (unpaired t test) compared with the corresponding mc-PAF-stimulated cells without pEF-C3. B, subcellular localizations of WT HA-hPAFR and P247A with or without exposure to mc-PAF were observed by immunofluorescence confocal microscopy. Twenty-four hours after transfection of HeLa cells with WT HA-hPAFR or P247A, the cells were treated with vehicle (ethanol) or mc-PAF (1 μm) for 24 h, then subjected to immunocytochemical analysis. Top, the early endosomes and HA-tagged hPAFRs were visualized with Alexa Fluor 546-conjugated transferrin (upper, red) and an anti-HA antibody (green), respectively, under permeabilized conditions. Bottom, localization of the receptors on the plasma membrane was confirmed under non-permeabilized conditions by merging with CellMaskTM Orange Plasma Membrane Marker (red). Under these conditions, P247A was not detected with or without mc-PAF stimulation. White bars indicate 10 μm. The data are representative of three independent experiments with identical results. C, Western blot analysis was performed using HeLa-WT, HeLa-P247A, and HeLa-mock cells. After incubation in the presence of Dox, these cells were treated with or without mc-PAF (1 μm) and chloroquine (30 μm) for 24 h, followed by preparation of cell lysates. Molecular standards are indicated at the left of the gel. The data are representative of three independent experiments with identical results.