FIGURE 7.
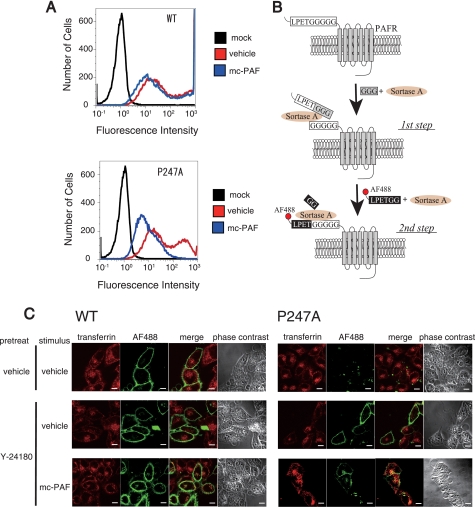
Aberrant trafficking of P247A after stimulation with mc-PAF. A, flow cytometric analysis was performed using HeLa-WT, HeLa-P247A, and HeLa-mock cells to evaluate the internalization of WT- and P247A-hPAFRs. After incubation in the presence of Dox, these cells were treated with Y-24180 (100 nm) for 12 h, followed by washes with PBS and incubation in DMEM (0.1% BSA) for 1 h to remove Y-24180. Thereafter, these cells were or were not stimulated with mc-PAF (3 μm) for 1 h. The data are representative of three independent experiments with identical results. B, N-terminal labeling of LPETGGGGG-tagged cell surface PAFRs with LPETGG-tethered Alexa Fluor 488 by sortase-A. The first step, preincubation of the cells expressing LPETGGGGG-tagged PAFRs with sortase-A and triglycine, allowed cleavage between the threonine and glycine of the LPETGGGGG tag, resulting in presentation of a pentaglycine tag at the N termini of the target PAFRs. In the following step, labeling of the target receptors with chemical probes was achieved by incubation of the cells with sortase-A and LPETGG-tethered Alexa Fluor 488. C, subcellular localizations of WT-hPAFR and P247A with or without stimulation of mc-PAF were observed by immunofluorescence confocal microscopy. After HeLa-WT and HeLa-P247A were cultured in the presence of Dox, these cells were pre-treated with or without Y-24180 (100 nm) for 12 h, followed by washes with PBS. The cell surface-trafficked receptors were specifically labeled with Alexa Fluor 488 by sortase-A as described in B. Thereafter, these cells were stimulated with or without mc-PAF (3 μm) for 1 h in the presence of Alexa Fluor 546-conjugated transferrin (50 μg/ml), and then subjected to immunocytochemical analysis. The early endosomes were visualized by Alexa Fluor 546-conjugated transferrin (red), and Alexa Fluor 488-labeled PAFRs are shown in green. White bars indicate 10 μm. The data are representative of three independent experiments with identical results.