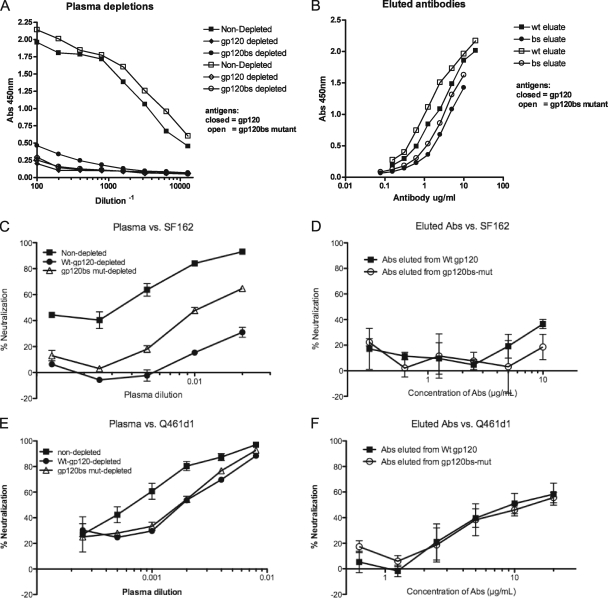
FIG. 4.
Contribution of antibodies to the CD4 binding site plasma from subject Q461. (A) Plasma from subject Q461 at 3.7 ypi was preincubated with magnetic beads coated with bovine serum albumin (BSA) (squares), wild-type SF162 gp120 (diamonds), or SF162gp120 containing mutations in the CD4 binding site (circles). The beads were removed, and the depleted plasma samples were tested for their ability to bind to wild-type SF162 gp120 (closed symbols) or CD4 binding site mutant gp120 (open symbols). The absorbance at 450 nm is shown on the y axis, with the serum dilution on the x axis. (B) Antibodies eluted from the wild-type SF162 gp120 beads (squares) or the CD4 binding site mutant (circles) were tested for their ability to bind to wild-type gp120 protein (closed) or gp120bs mutant protein (open). The absorbance at 450 nm is shown on the y axis, with the concentration of antibody on the y axis. (C) Plasma (represented in panel A) that was nondepleted (squares), depleted with wild-type SF162 gp120 protein (closed circles), or depleted with beads coated with CD4 binding site mutant gp120 protein (open triangles) were tested for their ability to neutralize SF162 pseudovirus. The percentage of neutralization is shown on the x axis, with the plasma dilution on the y axis. (D) The antibodies eluted from the Wt-gp120 or BS-mut gp120 beads, represented in panel B, were tested for their ability to neutralize the SF162 pseudovirus. (E) Ability of depleted plasma to neutralize Q461d1S pseudovirus, described in the legend for panel C. (F) Ability of antibodies eluted from Wt- or BS-mut-coated beads to neutralize Q461d1S pseudovirus, as described in the legend for panel D. All results are representative of two independent experiments; contribution of neutralization was calculated according to the AUC, as described in Materials and Methods.