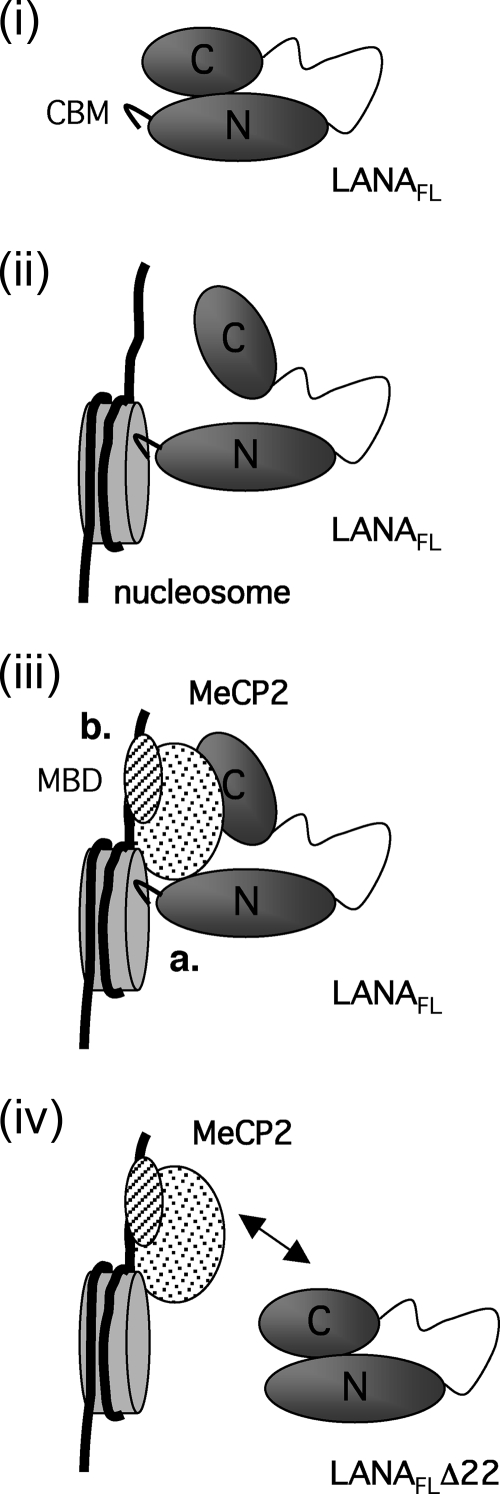
FIG. 8.
Schematics of possible multivalent interactions among LANA, core histones, and MeCP2. (i) Evidence suggests that LANA may be folded such that LANAN, which includes the CBM, is brought into proximity to LANAC. For simplicity, only one molecule of LANA is shown; however, LANA most likely exists as a stable dimer. (ii) Engagement of the CBM with a nucleosome may alter the conformation, making the C-terminal domain more accessible to secondary interactions. (iii) Interaction of LANAC with MeCP2 forms a relatively stable complex mediated by contacts between the CBM and the nucleosome core (a) and DNA and the MeCP2 MBD (b). As a consequence of these multivalent interactions, LANA accumulates with MeCP2 at the chromocenters. (iv) In the absence of the CBM, the LANA protein (LANAFLΔ22) shows reduced retention at the chromocenters. This may occur because the deletion alters the accessibility of the LANAC domain or increases the rate at which LANA exchanges with other interaction partners located in more euchromatic regions of the genome.