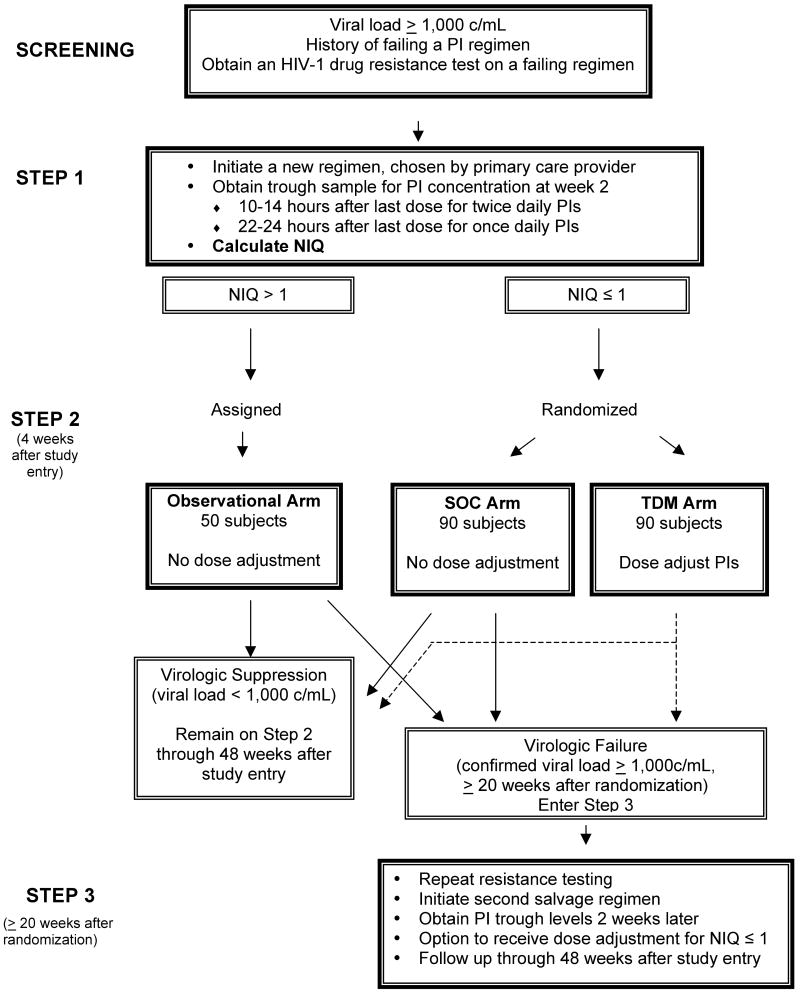
Figure 1. Study design of A5146.
The four phases of A5146 (screening, Steps 1, 2, and 3) are illustrated schematically. During the process of screening for study eligibility, a resistance test was obtained on the failing antiretroviral regimen, and used to design a new regimen. During Step 1, the new salvage antiretroviral regimen was begun at entry, and a PI trough sample was obtained 2 weeks later. The NIQ was calculated as outlined in the text, using the screening fold-change in IC50 for the PI(s) in the A5146 antiretroviral regimen and the week 2 trough PI concentration measured in patient plasma. Entry into Step 2 was dependent on the value of the NIQ. If it was ≤ 1, the subject was randomized to the TDM or SOC arms. If the NIQ was > 1, the subject entered Step 2 on the observational arm or discontinued study, if the accrual goal of the observational arm had been met. Subjects who developed virologic failure at or after 20 weeks post-randomization in any of the 3 arms of Step 2 could enter Step 3 to receive a repeat resistance test and TDM followed by dose escalation on a new salvage regimen. Maximum follow-up on study was 48 weeks after Step 1 entry.