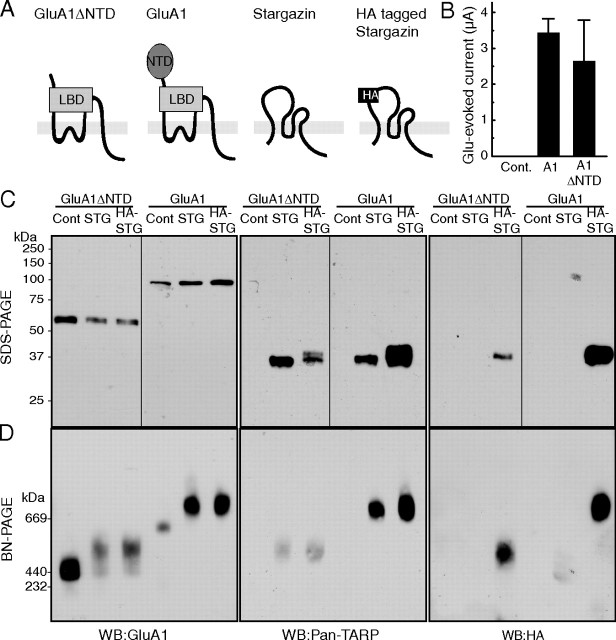
Figure 1.
AMPA receptors assemble with TARP/stargazin. A, Schematic domain structure of GluA1, GluA1 lacking the NTD (GluA1ΔNTD), stargazin, and HA-epitope-tagged stargazin (HA-stargazin). LBD, Ligand-binding domain. B–D, Oocytes were injected with cRNA of GluA1 (10 ng), GluA1ΔNTD (5 ng), stargazin, and HA-stargazin (0.25 ng), which was followed by measuring glutamate (100 μm)-evoked current with cyclothiazide (50 μm) by two-electrode voltage-clamp recording (B) and solubilization with Triton X-100 and SDS-PAGE (10%) (C), or BN-PAGE (4–8%) (D). Proteins were detected using an anti-GluA1 antibody (which recognizes the C terminus of GluA1), an anti-pan-TARP antibody (which recognizes the cytoplasmic domain of stargazin), and an anti-HA-epitope antibody. C, GluA1, stargazin (STG), and HA-stargazin were expressed at 100, 37, and 37 kDa, respectively, without detection of protein degradation on SDS-PAGE. D, GluA1 and GluA1ΔNTD formed homo-oligomers around 669 and 440 kDa, respectively, on BN-PAGE. The molecular weight of GluA1 and GluA1ΔNTD homo-oligomers shifted toward a higher molecular weight after coexpression with stargazin or HA-stargazin. The shifted bands of GluA1 and GluA1ΔNTD homo-oligomers were also recognized by an anti-pan-TARP antibody and an anti-HA antibody. WB, Western blotting; Cont, water-injected control.